Domain Electrochemistry Terms#
Electrochemistry Domain Ontology (ECHO)#
This reference module contains the core terms for the electrochemistry domain.
ACVoltammetrySignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_58a20764_c339_4856_983a_05092b5282e8
| elucidation | Sinusoidal potential waveform superimposed to a linear potential ramp. |
| subclassOf | ElectricPotentialSignal |
AcidicElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6592d8cc_4ce4_42ca_b010_6bfc4a8444d2
| altLabel | AcidicAqueousElectrolyte; AcidicSolution |
| elucidation | an aqueous electrolyte with a nominal pH values less than 7 |
| subclassOf | AqueousElectrolyte |
| subclasses | SulfuricAcidSolution, PhosphoricAcidSolution |
Note
typical alkaline electrolytes have a pH in the range 0 < x < 4
Example
HCl-H2O
ActivationEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d7f8cab9_b035_4ecd_be63_292672572526
| altLabel | ArrheniusActivationEnergy |
| elucidation | the activation energy in an arrhenius formulation |
| wikidataReference | https://www.wikidata.org/wiki/Q190474 |
| wikipediaReference | https://en.wikipedia.org/wiki/Activation_energy |
| iupacReference | https://doi.org/10.1351/goldbook.A00102 |
| subclassOf | MolarEnergy |
| subclasses | ActivationEnergyOfGuestDiffusivityInNegativeElectrodeActiveMaterial, ActivationEnergyOfElectrolyteConductivity, NegativeElectrodeActivationEnergyOfReaction, PostiveElectrodeActivationEnergyOfReaction, ActivationEnergyOfGuestDiffusivityInPositiveElectrodeActiveMaterial, ActivationEnergyOfChargeCarrierDiffusivityInElectrolyte |
ActivationEnergyOfChargeCarrierDiffusivityInElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_56de36fe_e8e1_486c_8d29_061ac8d28c13
| elucidation | the activation energy barrier in an Arrhenius expression for the diffusivity of lithium in an electrolyte |
| bpxKey | ['Parameterisation','Electrolyte','Diffusivity activation energy [J.mol-1]'] |
| cidemodKey | ['electrolyte','diffusion_constant','arrhenius','activation_energy'] |
| subclassOf | ActivationEnergy |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
ActivationEnergyOfElectrolyteConductivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8c16cb12_41c1_43bd_9e7c_2eea7b06a1f0
| elucidation | the activation energy barrier in an Arrhenius expression for the ionic conductivity of an electrolyte |
| bpxKey | ['Parameterisation','Electrolyte','Conductivity activation energy [J.mol-1]'] |
| cidemodKey | ['electrolyte','ionic_conductivity','arrhenius','activation_energy'] |
| subclassOf | ActivationEnergy |
ActivationEnergyOfGuestDiffusivityInNegativeElectrodeActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_86af4487_33c1_4562_a00b_3a8252ffe378
| elucidation | the activation energy barrier in an Arrhenius expression for the diffusivity of lithium in the negative electrode |
| bpxKey | ['Parameterisation','Negative electrode','Diffusivity activation energy [J.mol-1]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'diffusion_constant','arrhenius','activation_energy'] |
| subclassOf | ActivationEnergy |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
ActivationEnergyOfGuestDiffusivityInPositiveElectrodeActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4d69edda_d2fa_40b0_9c1e_52e08debf578
| elucidation | the activation energy barrier in an Arrhenius expression for the diffusivity of lithium in the positive electrode |
| bpxKey | ['Parameterisation','Positive electrode','Diffusivity activation energy [J.mol-1]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'diffusion_constant','arrhenius','activation_energy'] |
| subclassOf | ActivationEnergy |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
ActivationOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7fa406b0_512a_4d59_9e0c_5d8aba0103ae
| altLabel | ActivationPolarization |
| elucidation | part of the electrode polarization arising from a charge-transfer step of the electrode reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-05 |
| subclassOf | Overpotential |
ActiveElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9a823d64_9d10_4a29_9cbd_9bbdad7985bc
Note
the opposite of an inert electrode
ActiveMassLoading#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1eeb0e2a_229a_43f1_b197_348d475067ff
| elucidation | the mass of active material per unit area |
| subclassOf | MassLoading |
ActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_79d1b273_58cd_4be6_a250_434817f7c261
| altLabel | ActiveElectrochemicalMaterial; ElectrochemicallyActiveMaterial |
| elucidation | material that is oxidized or reduced at an electrode in an electrochemical cell |
| wikidataReference | https://www.wikidata.org/wiki/Q120907375 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-33; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-14 |
| restrictions | |
| subclassOf | ElectrochemicalComponent, ElectrochemicalMaterial |
Example
lithium iron phosphate
Example
zinc
ActiveMaterialMix#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_81833d8a_b03d_4250_be84_6385415beb01
| elucidation | blend containing a material which reacts chemically to produce electrical energy with other constituents and additives |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-34 |
| restrictions | |
| subclassOf | ElectrochemicalComponent |
Additive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a399f3f_b873_41f5_be1f_9b6df75cc30a
| elucidation | a substance added to something in small quantities to alter its properties |
| wikidataReference | https://www.wikidata.org/wiki/Q350176 |
| subclassOf | ElectrochemicalComponent |
| subclasses | ConductiveAdditive, ElectrolyteAdditive |
AdsorptionCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_214d925c_76c4_4f69_9afc_056a1ea82fc6
| elucidation | electric current that accompanies the adsorption of a species |
| iupacReference | https://goldbook.iupac.org/terms/view/A00159 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
AirElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8b40856f_1ca2_4137_9616_7fb624671909
| elucidation | a gas diffusion electrode in which the gas is air |
| restrictions | |
| subclassOf | GasDiffusionElectrode |
Note
The reaction occuring in an air electrode is typically the oxygen reduction reaction (ORR).
AlkalineElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_615cff2a_be95_4e65_9471_98db23f4c878
| altLabel | AlkalineAqueousElectrolyte; AlkalineSolution |
| elucidation | an aqueous electrolyte with a nominal pH greater than 7 |
| subclassOf | AqueousElectrolyte |
| subclasses | SodiumHydroxideSolution, PotassiumHydroxideSolution, AmmoniaSolution, LithiumHydroxideSolution |
Note
typical alkaline electrolytes have a pH in the range 13 < x < 15
Example
KOH-H2O
AlternatingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a53d6dee_1547_4854_a311_805b4d557298
| altLabel | AC |
| elucidation | periodic current where the amplitude alternates at a steady frequency between fixed minimum and maximum values, with the same duration at minimum and maximum |
| wikidataReference | https://www.wikidata.org/wiki/Q124164 |
| wikipediaReference | https://en.wikipedia.org/wiki/Alternating_current |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=131-11-24 |
| subclassOf | ElectricCurrentSignal |
| subclasses | SinusoidalCurrentWaveform |
AluminiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_952c8c3a_df21_4dd1_8d8c_380e43dc8c78
| elucidation | an electrode in which the primary active material consists of aluminium or aluminium compounds |
| subclassOf | ActiveElectrode |
AluminiumInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1c6cef85_811f_45d0_a0fd_2bc2d9369ea4
| elucidation | an insertion electrode in which the guest molecule is aluminium |
| subclassOf | InsertionElectrode |
AmbientCelsiusTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_abd08921_6f3b_4a48_811e_7685c58aec0b
| elucidation | the celsius temperature of the ambient environment |
| batteryArchiveLabel | Environmental_Temperature (C) |
| subclassOf | CelsiusTemperature |
AmbientThermodynamicTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_39a44af0_0e1a_4859_b550_bdabad64386e
| elucidation | the ambient thermodynamic temperature |
| subclassOf | ThermodynamicTemperature |
AmmoniaSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f9e2e676_5cd1_4e22_a776_af45838d4027
| altLabel | AqueousAmmoniaSolution |
| elucidation | a solution of ammonia (NH3) dissolved in water (H2O) |
| subclassOf | AlkalineElectrolyte |
Note
the solution can also be made by dissolving ammonium hydroxide (NH4OH) in water
AmmoniumChlorideSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3f67016b_32b9_4419_a3f8_a5ffb5e92538
| altLabel | AqueousAmmoniumChlorideSolution |
| elucidation | a solution of ammonium chloride (NH4Cl) dissolved in water (H2O) |
| subclassOf | NearNeutralElectrolyte |
AmperePerAmpereHour#
IRI: https://w3id.org/emmo/domain/electrochemistry#AmperePerAmpereHour
| elucidation | the unit for C-Rate in electrochemistry and related domains |
| subclassOf | CRateUnit |
AmplitudeOfAlternatingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_10eb778d_da60_4832_a355_4ee74baea650
| elucidation | half the peak-to-peak amplitude of a sinusoidal alternating current |
| iupacReference | https://doi.org/10.1351/goldbook.A00310 |
| subclassOf | ElectricCurrent, ElectrochemicalControlQuantity |
AmplitudeOfAlternatingVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f591a444_89d6_4093_836d_7d53895edce4
| elucidation | half of the peak-to-peak amplitude of a periodic voltage perturbation within a single time period, with respect to a reference potential value |
| iupacReference | https://doi.org/10.1351/goldbook.A00311 |
| subclassOf | ElectricPotential, ElectrochemicalControlQuantity |
Note
This term should denote half of the peak-to-peak amplitude. Peak-to-peak and r.m.s. amplitudes should be so specified.
AnionExchangeMembrane#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4586bc75_3370_4190_bbf8_33d2e0a77aa4
| elucidation | a selective barrier that permits the passage of anions while obstructing the passage of cations |
| wikipediaReference | https://en.wikipedia.org/wiki/Anion-exchange_membrane |
| subclassOf | IonExchangeMembrane |
AnnularWorkingElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3a77b5e7_9646_4154_bf8f_5f798989e5f3
| altLabel | RingElectrode |
| elucidation | a working electrode in the shape of a ring used in a rotating ring disk electrode (RRDE). |
| subclassOf | WorkingElectrode |
Anode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b6319c74_d2ce_48c0_a75a_63156776b302
| elucidation | by convention, cell electrode at which an oxidation reaction occurs |
| etymology | From Greek anodos (‘way up’). Chosen to denote the electrode associated with the ‘upward’ path of current by convention; later anchored by usage to the site of oxidation.Coined by Michael Faraday with guidance from William Whewell and Whitlock Nicholl. |
| wikidataReference | https://www.wikidata.org/wiki/Q181232 |
| wikipediaReference | https://en.wikipedia.org/wiki/Anode |
| dbpediaReference | https://dbpedia.org/page/Anode |
| iupacReference | https://goldbook.iupac.org/terms/view/A00370 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-27 |
| subclassOf | Electrode |
Note
The anode is the negative electrode during discharge and the positive electrode during charge.
Warning
The term "anode" is often used interchangably with "negative electrode" even though it only applies while the cell is being discharged. When the cell is being charged the positive electrode becomes the anode.
Important
IEC and IUPAC recommend defining anode/cathode by oxidation/reduction rather than by polarity or charge sign.
AnodicOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_565c0b10_70fe_441a_b76a_b9a8e08ca7b7
| altLabel | AnodicPolarization |
| elucidation | electrode polarization associated with an electrochemical oxidation reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-06 |
| subclassOf | ReactionOverpotential |
AnodicPolarisation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_28213033_4c74_441c_81c4_a0cad05f9eb6
| altLabel | AnodicPolarization |
| elucidation | electrode polarization associated with an anodic reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-17 |
| subclassOf | ElectrodePolarisation |
AnodicReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a0580fa9_5073_44af_b33e_7adbc83892d0
| altLabel | Electrooxidation; ElectrooxidationReaction |
| elucidation | electrode reaction in which oxidation occurs at the anode |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-09 |
| subclassOf | ElectrodeReaction, Oxidation |
| subclasses | Electrodissolution |
Anolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_865a40fc_2187_4549_a7e1_37aa2458448f
| elucidation | electrolyte on the anode side of an electrochemical cell that is divided into compartments |
| etymology | From Greek anodos ('way up') + lytēs (, ‘looser’) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-19 |
| subclassOf | Electrolyte |
Note
Electrolyte solution in the anodic compartment of an electrolysis cell or galvanic cell, i.e., in that part of the cell where the anode is placed.
AnolyteCompartment#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fe51c981_a5c7_414d_90c9_3f44c7e75154
| elucidation | an internal compartment of the electrochemical flow cell through which anolyte flows |
| subclassOf | Component |
Note
AnolyteTank holds the anolyte typically outside of the eletrochemical cell, where the term AnolyteCompartment describes a space inside the electrochemical flow cell.
AnolyteTank#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_38755e67_9d3b_4a82_bd8d_ef40a70379c1
| elucidation | a tank for holding anolyte |
| subclassOf | Component |
Note
usually used in a flow cell system
AqueousElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b812e9d0_7c58_4455_b3e7_6847f10c8e8a
| altLabel | AqueousElectrolyticSolution |
| elucidation | an ion-transport medium, which may be immobilized, in which water is the solvent. |
| wikidataReference | https://www.wikidata.org/wiki/Q120906632 |
| dbpediaReference | https://dbpedia.org/page/Aqueous_solution |
| subclassOf | AqueousSolution, ElectrolyteSolution |
| subclasses | AlkalineElectrolyte, NearNeutralElectrolyte, AcidicElectrolyte |
AreicCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bcb33f7e_5573_4bc2_b636_4ea313a9dd3a
| altLabel | ArealCapacity |
| elucidation | quotient of the capacity of a battery cell or battery by its plane area. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-20 |
| subclassOf | ISQDerivedQuantity, ElectrochemicalPerformanceQuantity |
AsymmetricMembrane#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b2d11f0d_c1b0_4476_8d17_03b73d31e01f
| elucidation | membrane constituted of two or more structural planes of non-identical morphologies |
| wikidataReference | https://www.wikidata.org/wiki/Q120965018 |
| iupacReference | https://doi.org/10.1351/goldbook.AT06862 |
| subclassOf | Separator |
| subclasses | BilayerMembrane |
BaselineCellVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_269ddd97_1437_4545_b272_0df75a12c68a
| elucidation | In electrochemical measurements, the voltage of an electrochemical cell to which a voltage signal is superimposed. |
| subclassOf | CellVoltage, ElectrochemicalControlQuantity |
Example
in EIS, an alternating voltage signal is superimposed to a baseline voltage, which can be the open circuit potential or a fixed cell voltage
BellevilleWasher#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_98d5101e_cd11_4a0d_b856_6fcd8aad132e
| elucidation | a type of spring, in the form of a conical washer |
| wikidataReference | https://www.wikidata.org/wiki/Q3056595 |
| subclassOf | Spring |
BilayerMembrane#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f1c7eacb_9f21_4100_925c_3974f266e06f
| elucidation | an asymmetric membrane composed of two layers, typically with different chemical properties or functional roles |
| subclassOf | AsymmetricMembrane |
BimetallicElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_86be0987_5e21_43ec_b975_8f679999d328
| elucidation | electrode containing two different metals (e.g. platinum and ruthenium) on its surface (e.g. to modify its electrocatalytic properties) |
| subclassOf | BlendedActiveElectrode, MetalElectrode |
BimetallicOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4839708a_3864_47eb_b719_373ff8874c61
| elucidation | an electrode consisting of two metal oxide active materials |
| subclassOf | BlendedActiveElectrode, MetalOxideElectrode |
| subclasses | LithiumManganeseOxideLithiumIronPhosphateElectrode, IndiumTinOxideElectrode, LithiumNickelManganeseOxideLithiumIronPhosphateElectrode, LithiumNickelMananeseCobaltOxideLithiumManganeseOxideElectrode, LithiumNickelManganeseCobaltOxideLithiumCobaltOxideElectrode |
BinaryElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4e02d727_07fe_41fd_886c_041317342086
| elucidation | an electrolyte consisting of anions and cations with equal absolute charge numbers. |
| subclassOf | Electrolyte |
Example
KCl (1:1), MgSO4 (2:2)
Binder#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_68eb5e35_5bd8_47b1_9b7f_f67224fa291e
| altLabel | ElectrodeBinder |
| elucidation | material or substance that holds or draws other materials together to form a cohesive whole |
| wikidataReference | https://www.wikidata.org/wiki/Q863583 |
| wikipediaReference | https://en.wikipedia.org/wiki/Binder_(material) |
| subclassOf | ElectrochemicalComponent |
Example
CMC, PVDF
BinderSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bed8f6c8_18d3_4af0_a0f3_d83bfa80498b
| elucidation | a liquid solution comprising binder dispersed in a solvent |
| subclassOf | LiquidSolution |
BinderSolutionMixing#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_91e931b3_9bc8_4490_8a9c_3202fc8338bd
| elucidation | the process of mixing binder components to create a binder solution |
| subclassOf | Mixing |
BismuthBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eb09ca25_90c9_4b55_9165_76fbf7fb5a46
| elucidation | an electrode in which the primary active material consists of bismuth or bismuth compounds. |
| subclassOf | ActiveElectrode |
BlendedActiveElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5186239a_2af7_4dbf_92ca_22e8e583c528
| elucidation | an active electrode with a blend of two or more active materials |
| subclassOf | ActiveElectrode |
| subclasses | SiliconOxideGraphiteElectrode, BimetallicOxideElectrode, SiliconGraphiteElectrode, BimetallicElectrode |
BockrisDevanathanMuellerModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4bdd6359_1422_4c50_ac0c_5d8042dd65fc
| altLabel | BDM |
| elucidation | a model for the double layer that includes the action of the solvent on the surface |
| subclassOf | DoubleLayerModel |
Note
proposed by John Bockris, M. A. V. Devanathan, and Klaus Mueller in 1963
BodePlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e0b57b09_68ee_4687_a901_bfb599421972
| altLabel | BodeDiagram |
| elucidation | plots of the phase angle and the magnitude of the impedance vs. the logarithm of perturbation frequency at all the frequencies tested in an impedimetry measurement |
| wikidataReference | https://www.wikidata.org/wiki/Q245627 |
| wikipediaReference | https://en.wikipedia.org/wiki/Bode_plot |
| subclassOf | ElectrochemicalPlot |
BoostCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_02aefb7a_d6ce_4b6e_b854_f7b3d641f670
| altLabel | FastCharging |
| elucidation | accelerated charge applied at greater than normal values of electric currents or of voltages (for a particular design) during a short time interval |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-37 |
| subclassOf | Charging |
BoundaryCondition#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f2f36f22_3738_49dd_b43b_7469db6675df
| elucidation | a property whose value is controlled on some surface or domain |
| subclassOf | ControlProperty |
BruggemanCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5c34b3b5_c9c4_477d_809a_3f682f995aa9
| elucidation | quantity that is used to estimate effective transport coefficients in porous media |
| subclassOf | PureNumberQuantity |
ButlerVolmerEquation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d48ea516_5cac_4f86_bc88_21b6276c0938
| altLabel | ButlerVolmerApproximation; ErdeyGruzVolmerEquation |
| elucidation | a phenomenological model for electrode kinetics, describing the relation between the electrode current from an electrochemical charge-transfer reaction and the surface overpotential of the electrode |
| wikidataReference | https://www.wikidata.org/wiki/Q903846 |
| wikipediaReference | https://en.wikipedia.org/wiki/Butler%E2%80%93Volmer_equation |
| dbpediaReference | https://dbpedia.org/page/Butler%E2%80%93Volmer_equation |
| subclassOf | ElectrochemicalRelation |
Note
i = i0 * (exp(alpha*n*F*eta/(R*T)) – exp(-(1-alpha)*n*F*eta/(R*T)))
C#
IRI: https://w3id.org/emmo/domain/electrochemistry#C
| elucidation | the unit for C-Rate in electrochemistry and related domains |
| subclassOf | CRateUnit |
CRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e1fd84eb_acdb_4b2c_b90c_e899d552a3ee
| altLabel | CapacityRate |
| elucidation | [an indicator of the] electric current at which a secondary cell or battery is charged |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
| subclasses | LowerCRateLimit, DischargingCRate, ChargingCRate, FinishingCRate |
CRateTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_99b2b3ad_8efc_48ee_a630_6d805a47efdc
| elucidation | a process that assesses the performance of an electrochemical device at different charge and discharge rates to understand its behavior under various current loads |
| restrictions | |
| subclassOf | ElectrochemicalTesting |
CRateUnit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_67930f7b_ad4c_4b21_9b2e_a000e5c153ae
| elucidation | the class of units for describing the C-Rate in electrochemistry and related domains |
| restrictions |
|
| subclassOf | SIDimensionalUnit |
| subclasses | C, AmperePerAmpereHour |
CadmiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dd4c5ffa_6228_41d8_8a44_a40a2b22723e
| elucidation | an electrode in which the primary active material consists of cadmium or cadmium compounds. |
| subclassOf | ActiveElectrode |
CaesiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7cc8b738_3462_4592_ba83_951a8d50fef7
| elucidation | an electrode in which the primary active material consists of caesium or caesium compounds |
| subclassOf | ActiveElectrode |
CalciumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4f2348dd_d9ea_4448_af8c_a4a38f3d04b4
| elucidation | an electrode in which the primary active material consists of calcium or calcium compounds |
| subclassOf | ActiveElectrode |
CalciumInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_90103be0_9096_4f98_89c7_b5db01197858
| elucidation | an insertion electrode in which the guest molecule is calcium |
| subclassOf | InsertionElectrode |
CalenderedCoatingThickness#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f9e1c862_c510_4b11_9141_bc91045df817
| elucidation | thickness of the coating after a calendering process |
| subclassOf | CoatingThickness |
CalenderedDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_520995f8_ec9c_4b3c_bb64_2cd691947379
| elucidation | density after calendering |
| subclassOf | Density, ElectrochemicalQuantity |
Capacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_791c1915_a791_4450_acd8_7f94764743b5
| altLabel | ChargeCapacity; ElectricChargeCapacity; StorageCapacity |
| elucidation | electric charge which an energy storage device can deliver under specified conditions |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| hiddenLabel | AH; Amp-hr; Capacity/mAh; mAmp-hr |
| maccorLabel | amp-hr |
| newareLabel | Capacity(mAh) |
| indigoLabel | cell_coulomb_count_c |
| novonixLabel | Capacity (Ah) |
| subclassOf | ElectricCharge, ElectrochemicalQuantity, ISQDerivedQuantity |
| subclasses | ChargingCapacity, DischargingCapacity, ResidualCapacity, NetCapacity, RatedCapacity, StepCapacity, CumulativeCapacity |
CapacityCalculation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9d97c7ff_b0c7_4ba2_a3cb_c6509b6798a8
| elucidation | data processing procedure that determines the capacity based on time data and electric current data |
| restrictions | |
| subclassOf | MeasurementDataPostProcessing |
CapacityFade#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e3d3d21c_cb9a_498c_bdb0_63c964f0d3c6
| altLabel | CapacityLoss |
| elucidation | a degradation phenomena in electrochemical devices in which the practicaclly achieveable capacity of the cell is less than the measured capacity at the beginning of life. |
| wikidataReference | https://www.wikidata.org/wiki/Q16851742 |
| wikipediaReference | https://en.wikipedia.org/wiki/Capacity_loss |
| restrictions | |
| subclassOf | ElectrochemicalDegradationPhenomenon |
| subclasses | SelfDischarging |
CapacityTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_137dc19f_a3af_49af_971f_743d27e09f43
| elucidation | a process that measures the total amount of charge an electrochemical device can store and deliver under specified conditions |
| restrictions | |
| subclassOf | Electrochemical |
CarbonBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f2c33088_224f_4fdb_857a_7cb62e3dddca
| elucidation | an electrode in which the primary active material consists of carbon or carbon compounds |
| subclassOf | Electrode |
| subclasses | HardCarbonElectrode, CarbonMonofluorideElectrode, GraphiteElectrode |
Note
often represented in IEC cell designations by the letter I
Note
this class is intended to enable designations based on IEC recommendations
CarbonCloth#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_85c4b478_645a_459e_8431_5a9d864ca02e
| elucidation | a woven fabric made from carbon fibers, offering flexibility, high electrical conductivity, and durability, commonly used in high-performance fuel cells |
| subclassOf | ManufacturedMaterial |
CarbonFelt#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1c530688_ee63_4683_b38f_80cce7ca75c1
| elucidation | a non-woven mat of randomly oriented carbon fibers, providing excellent thermal insulation, chemical resistance, and high surface area for various industrial and electrochemical applications |
| subclassOf | ManufacturedMaterial |
CarbonInkElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ec6f3d6f_bdf5_418f_9314_3ef2ff528103
| elucidation | development of a carbon paste electrode that is screen printed using a carbon/polymer mixture of suitable composition |
| wikidataReference | https://www.wikidata.org/wiki/Q120907443 |
| subclassOf | CarbonPasteElectrode |
Note
These electrodes can easily be chemically modified by the incorporation of reagents (electrocatalysts, redox mediators, complexation agents, enzymes, etc.) and by the attachment of micro- or nanoparticles to prepare electrochemical sensors for analytical applications.
Note
Typical composition of a carbon ink expressed as mass fractions is graphite powder 60 % and polystyrene 40 %.
CarbonMonofluorideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5c0fdc09_166e_40a6_ad74_be66f0db51bc
| elucidation | electrode in which the active material is carbon monofluoride |
| restrictions | |
| subclassOf | ActiveElectrode, CarbonBasedElectrode |
Note
the combination with lithium metal and an organic electrolyte is designated using IEC electrochemical system letter code B
CarbonPaper#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cd615729_8240_487a_a619_cc94656731f2
| elucidation | a thin, flat material composed of compressed carbon fibers, used in fuel cells and other electrochemical devices for its high electrical conductivity and gas permeability |
| subclassOf | ManufacturedMaterial |
CarbonPasteElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b0a0dddb_d942_4af2_b6a7_d7165f4253f1
| altLabel | CPE |
| elucidation | Electrode of a composite of carbon powder and a pasting liquid (including mineral oil, Nujol, bromoform, bromonaphthalene). |
| wikidataReference | https://www.wikidata.org/wiki/Q5037987 |
| wikipediaReference | https://en.wikipedia.org/wiki/Carbon_paste_electrode |
| subclassOf | InertElectrode |
| subclasses | CarbonInkElectrode |
Note
The CPE was first described by Adams in 1958. A CPE is typically housed in a Teflon holder, contacted by a conductive wire (occasionally a piston is used to renew the surface by extrusion of the used paste). Problems are long-term stability of the paste and that the binder can influence its properties.
Note
These electrodes can easily be chemically modified by the incorporation of reagents (electrocatalysts, redox mediators, complexation agents, enzymes, etc.) and by the attachment of micro- or nanoparticles to prepare electrochemical sensors for analytical applications.
CardonDioxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_573be62a_5aae_4984_9a51_7c50845493df
| elucidation | a gas diffusion electrode in which the active material is carbon dioxide (CO2) |
| subclassOf | GasDiffusionElectrode |
Case#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1aec4cc0_82d5_4042_a657_ed7fe291c3d8
| altLabel | Can; Container; Housing |
| elucidation | container for the plate pack or packs and electrolyte of a cell or cells made of a material impervious to the electrolyte |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-14 |
| subclassOf | ElectrochemicalComponent |
| subclasses | PrismaticCase, PouchCase, RoundCase, SwagelokCase |
Catalyst#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8ab1e656_38ff_48e6_ab09_293d76bc9044
| elucidation | substance that increases chemical reaction speed, and which is conserved after the reaction |
| wikidataReference | https://www.wikidata.org/wiki/Q12385831 |
| subclassOf | Agent |
| subclasses | Electrocatalyst |
CatalyticCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c55bcb85_b7b8_4e67_8a78_9a42fe25b6cf
| elucidation | faradaic current measured in a solution containing two electroactive substances, A and B, that exceeds the sum of the faradaic currents that would be obtained for A and B separately under the same experimental conditions |
| iupacReference | https://doi.org/10.1351/goldbook.C00889 |
| subclassOf | FaradaicCurrent |
Cathode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_35c650ab_3b23_4938_b312_1b0dede2e6d5
| elucidation | by convention, cell electrode at which, a reduction reaction occurs |
| etymology | From Greek kathodos (‘way down’). Intended to name the electrode on the ‘downward’ path of current by convention; in modern usage the site of reduction. Coined by Michael Faraday with support from William Whewell and Whitlock Nicholl. |
| wikidataReference | https://www.wikidata.org/wiki/Q175233 |
| wikipediaReference | https://en.wikipedia.org/wiki/Cathode |
| dbpediaReference | https://dbpedia.org/page/Cathode |
| iupacReference | https://goldbook.iupac.org/terms/view/C00905 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=151-13-03; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-28 |
| subclassOf | Electrode |
Note
The cathode is the positive electrode during discharge and the negative electrode during charge.
Warning
The term "cathode" is often used interchangably with "positive electrode" even though it only applies while the cell is being discharged. When the cell is being charged the negative electrode becomes the cathode.
Important
IEC and IUPAC recommend defining anode/cathode by oxidation/reduction rather than by polarity or charge sign.
CathodeElectrolyteInterphase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f8e0d532_cf44_403c_9188_e00ee161a3c1
| elucidation | a type of solid electrolyte interface that forms on the surface of the electrode designated as the "cathode" or positive electrode |
| subclassOf | SolidElectrolyteInterphase |
Note
used to distinguish the interphase on the cathode surface from that on the anode, which is typically called the solid electrolyte interphase (SEI)
CathodicOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0853b072_3b80_4864_8147_24ce35407ade
| altLabel | CathodicPolarization |
| elucidation | electrode polarization associated with an electrochemical reduction reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-07 |
| subclassOf | ReactionOverpotential |
CathodicPolarisation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_187326b9_1089_4122_8e7e_1a0bcba210a1
| altLabel | CathodicPolarization |
| elucidation | electrode polarization associated with a cathodic reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-18 |
| subclassOf | ElectrodePolarisation |
CathodicProtection#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c936bfbe_7a0c_4185_a317_db1ce2c3c38c
| elucidation | electrochemical immunity produced by an appropriate cathodic polarization. |
| wikidataReference | https://www.wikidata.org/wiki/Q15152527 |
| wikipediaReference | https://en.wikipedia.org/wiki/Cathodic_protection |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-05 |
| subclassOf | IntentionalAgency, ElectrochemicalImmunity |
CathodicReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f4a1323a_ce2b_4c1a_b89d_c80170110ed6
| altLabel | Electroreduction; ElectroreductionReaction |
| elucidation | electrode reaction in which reduction occurs at the cathode |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-12; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-10 |
| subclassOf | Reduction, ElectrodeReaction |
| subclasses | Electrodeposition |
Note
electrode reaction involving an electrochemical reduction
Note
electrode reaction, where the energy of electrons in the working electrode is higher than the LUMO orbital of the substrate
Catholyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_15b852b5_19cc_49ab_849f_7df6175fb2be
| elucidation | electrolyte on the cathode side of an electrochemical cell that is divided into compartments. |
| etymology | From Greek kathodos ('way down') + lytēs (, ‘looser’) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-18 |
| subclassOf | Electrolyte |
Note
Electrolyte solution in the cathodic compartment of an electrolysis cell or galvanic cell, i.e., in that part of the cell where the cathode is placed.
CatholyteCompartment#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c2e3af24_a76b_4bab_9b48_57f21455d721
| elucidation | an internal compartment of the electrochemical flow cell through which catholyte flows |
| subclassOf | Component |
Note
CatholyteTank holds the anolyte typically outside of the eletrochemical cell, where the term CatholyteCompartment describes a space inside the electrochemical flow cell.
CatholyteTank#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9b6853e7_3412_463d_b5a9_82c14acebf7d
| elucidation | a tank for holding catholyte |
| subclassOf | Component |
Note
usually used in a flow cell system
CationExchangeMembrane#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8b6192f2_0b45_46fb_8316_f75d93953428
| elucidation | a selective barrier that permits the passage of cations while obstructing the passage of anions |
| subclassOf | IonExchangeMembrane |
CellBaffle#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_328c6e36_3706_4d92_abae_432fa3adb2a0
| elucidation | internal component used to reduce the amount of electrolyte loss due to electrolyte spray being carried in the gas and/or by electrolyte movement |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-13 |
| subclassOf | Component |
Note
a cell baffle has a second function in protecting the plate pack from damage by objects inserted through the filling hole
CellCan#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4a5660bd_1c1a_40e5_8a41_463c720d3903
| elucidation | cell container which is usually metallic and typically, but not exclusively, cylindrical |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-13 |
| subclassOf | ElectrochemicalComponent |
CellCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_637ee9c4_4b3f_4d3a_975b_c0572dfe53ce
| elucidation | electric current flowing to or from an electrochemical cell or device |
| hiddenLabel | /mA; Amps; Current/mA; mAmps |
| maccorLabel | amps |
| batteryArchiveLabel | Current (A) |
| newareLabel | Current(mA) |
| indigoLabel | cell_current_a |
| arbinLabel | current |
| novonixLabel | Current (A) |
| biologicLabel | I/mA |
| subclassOf | InstantaneousCurrent |
| subclasses | DischargingCurrent, CurrentScanRate, ChargingCurrent, ShortCircuitCurrent |
CellLid#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1e33e37e_d7c9_4701_ba6d_a09456a13aaf
| elucidation | part used to close the case normally having holes for filling, topping-up, gas escape, terminals, etc. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-15 |
| subclassOf | ElectrochemicalComponent |
CellPackaging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6a2d9bd3_3695_4827_b3f7_3bba9b947129
| elucidation | the process of inserting a dry cell assembly into a container |
| subclassOf | IntentionalAgency |
| subclasses | PrismaticCellPackaging, CylindricalCellPackaging, PouchCellPackaging |
CellPolarisationPotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_265bb4d6_5eec_40f6_a3fa_59b3bd08e9af
| altLabel | CellPolarizationPotential |
| elucidation | sum of the absolute values of the potential differences resulting from anodic and cathodic polarizations of an electrochemical cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-12 |
| subclassOf | CellVoltage |
CellVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4ebe2ef1_eea8_4b10_822d_7a68215bd24d
| altLabel | AppliedPotential |
| elucidation | voltage between the terminals of an electrochemical cell |
| iupacReference | https://doi.org/10.1351/goldbook.A00424 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-10 |
| hiddenLabel | Ecell/V; Voltage/V; Volts |
| maccorLabel | volts |
| batteryArchiveLabel | Voltage (V) |
| newareLabel | Voltage(V) |
| indigoLabel | cell_voltage_v |
| arbinLabel | voltage |
| novonixLabel | Potential (V) |
| biologicLabel | Ecell/V |
| subclassOf | Voltage, ElectrochemicalQuantity |
| subclasses | BaselineCellVoltage, DischargingVoltage, ChargingVoltage, OpenCircuitVoltage, CellPolarisationPotential, PulseVoltage |
Note
Cell voltage fluctuates based on factors such as applied current, internal resistance, temperature, and composition.
Note
Cell voltage represents the instantaneous electrical potential difference between the positive and negative electrodes of an electrochemical cell. It is a key parameter for monitoring battery performance, estimating state-of-charge, and diagnosing potential failures.
CelsiusTemperatureData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_efaca8db_a3e0_4188_9c9b_ed0037966725
| elucidation | celsius temperature data, usually resulting from an electrochemical measurement process |
| restrictions | |
| subclassOf | Vector, RawData |
CelsiusTemperatureMeasurement#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bea04148_10d9_4eda_9cd5_8f609d7e9ff8
| elucidation | measurement of temperature |
| restrictions | |
| subclassOf | Measurement |
CelsiusTemperatureMeasurementResult#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2dea31c2_5061_4464_ab76_5336bef23629
| elucidation | a measurement of the value of the temperature in units of degree Celsius |
| subclassOf | MeasurementResult |
ChargeCarrier#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d1042a12_e4be_4992_86cb_59420ef4e05c
| elucidation | a generic denomination of particles or quasiparticles responsible for electric charge transport. |
| wikidataReference | https://www.wikidata.org/wiki/Q865807; https://www.wikidata.org/wiki/Q865807 |
| wikipediaReference | https://en.wikipedia.org/wiki/Charge_carrier |
| subclassOf | ElectrochemicalComponent |
Example
electron, hole, ion
ChargeCarrierDiffusivityInElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4c274506_af5b_4ef1_8217_829ffd459f28
| elucidation | the diffusivity of lithium in an electrolyte |
| bpxKey | ['Parameterisation','Electrolyte','Diffusivity [m2.s-1]'] |
| cidemodKey | ['electrolyte','diffusion_constant','value'] |
| subclassOf | Diffusivity |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
ChargeCarrierTransportNumber#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e3e78df2_d568_4ab7_8c0d_d3a2ee3ae282
| elucidation | transport number of lithium ion in electrolyte |
| bpxKey | ['Parameterisation','Electrolyte','Cation transference number'] |
| cidemodKey | ['electrolyte','transference_number','value'] |
| subclassOf | IonTransportNumber |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
ChargeEfficiency#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a5962e05_466d_46a4_8951_bea59d7326e5
| elucidation | ratio of the electric charge discharged from a secondary battery to the electric charge provided during the preceding charge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-39 |
| subclassOf | RatioQuantity, ElectrochemicalPerformanceQuantity |
ChargeRetention#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_49efb72a_f8e6_4f50_acac_975302200d47
| altLabel | CapacityRetention |
| elucidation | ability of a cell or battery to retain capacity on open circuit under specified conditions. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-35 |
| subclassOf | ElectrochemicalProperty |
ChargeTimePlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_46676855_68b0_4096_ac6c_35400111d46d
| altLabel | ChargeTimeCurve; ElectricChargeTimeCurve; ElectricChargeTimePlot; QtCurve |
| elucidation | plot of the time-dependent amount of electric charge passed through an electrochemical system due to the application of a electric potential |
| subclassOf | ElectrochemicalPlot |
ChargeTransferCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a4dfa5c1_55a9_4285_b71d_90cf6613ca31
| elucidation | the fraction of the electrostatic potential energy affecting the reduction rate in an electrode reaction, with the remaining fraction affecting the corresponding oxidation rate |
| wikipediaReference | https://en.wikipedia.org/wiki/Charge_transfer_coefficient |
| subclassOf | RatioQuantity, ElectrochemicalKineticQuantity |
Charging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a9873d3d_945b_40ba_b9cd_8dfb64cec88f
| altLabel | ElectrochemicalCharging |
| elucidation | operation during which a secondary cell or battery is supplied with electric energy from an external circuit which results in chemical changes within the cell and thus the storage of energy as chemical energy. |
| wikidataReference | https://www.wikidata.org/wiki/Q11388109 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-27 |
| restrictions | |
| subclassOf | Procedure |
| subclasses | Overcharging, BoostCharging, FloatCharging, ConstantCurrentCharging, TwoStepCharging, EqualizationCharging |
ChargingCRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a7dc73e2_d4aa_4dfc_8b4d_cb611f1501fb
| elucidation | [an indicator of the] electric current at which a secondary cell or battery is charged |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-45 |
| subclassOf | CRate |
Note
the charge rate is expressed as the reference current It = Cr/n where Cr is the rated capacity declared by the manufacturer and n is the time base in hours for which the rated capacity is declared
ChargingCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_10763eb0_dbc9_4d34_bd1a_7b8996590d45
| altLabel | ChargeCapacity |
| elucidation | electric charge which an energy storage device can deliver under specified charging conditions |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| hiddenLabel | AH-IN; CapC; CapC/mAh; Q charge/mA.h |
| maccorLabel | wf chg cap |
| batteryArchiveLabel | Charge_Capacity (Ah) |
| newareLabel | Capacitance_Chg(mAh) |
| arbinLabel | charge_capacity |
| biologicLabel | Q charge/mA.h |
| subclassOf | Capacity |
| subclasses | ConstantCurrentChargingCapacity |
ChargingConstantCurrentPercentage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7f073272_8925_4344_995c_a5a6dd1fcde6
| altLabel | ConstantCurrentChargePercentage |
| elucidation | the percentage of the total charge capacity that is obtained during a constant current charge process |
| hiddenLabel | CC-Per; CC-Per/% |
| subclassOf | RatioQuantity |
ChargingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_79551e01_4bc6_4292_916e_08fe28a84600
| elucidation | electric current applied to an electrochemical device during a charging process |
| subclassOf | CellCurrent, ElectrochemicalQuantity |
ChargingData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bf7bfbcb_0698_47af_8678_af92b2f10414
| elucidation | data that is obtained from experiment or simulation during a charging process |
| subclassOf | Dataset |
ChargingEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2ab7af60_da58_4243_b3bc_cbb2155cac53
| altLabel | ChargeEnergy |
| elucidation | energy delivered by a deviced under some specific charge conditions |
| hiddenLabel | Energy charge/W.h; EnergyC; EnergyC/mWh; WH-IN |
| maccorLabel | wf chg e |
| batteryArchiveLabel | Charge_Energy (Wh) |
| newareLabel | Engy_Chg(mWh) |
| arbinLabel | charge_energy |
| biologicLabel | Energy charge/W.h |
| subclassOf | StoredEnergy |
ChargingEnergyDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_31a74e23_bb07_41d0_bb8f_1d8cca157503
| elucidation | the energy density of a device obtained during a charging process |
| subclassOf | EnergyDensity |
ChargingPRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_93f000d8_efc1_4364_896c_c9ebc4c7ce3a
| elucidation | an indicator of the electric power at which an electrochemical device is charged |
| subclassOf | PRate |
ChargingSpecificCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4882cf2f_aab7_4a3a_a103_7f56b55fbed3
| altLabel | SpecificChargeCapacity |
| elucidation | quotient of the capacity of a cell or battery [ or electrode or active material ] obtained during a charge process by its mass. |
| hiddenLabel | SpeCapC; SpeCapC/mAh/g |
| subclassOf | SpecificCapacity |
ChargingSpecificEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5e4490b8_c1dd_4e00_980b_c484e1bf4904
| altLabel | SpecificEnergyCharge |
| elucidation | the specific energy of an electrochemical device obtained during a charge process |
| hiddenLabel | SpeEnergyC; SpeEnergyC/mWh/g |
| subclassOf | SpecificEnergy |
ChargingVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_79a9e1be_35b0_4c3c_8087_b5f967ca0e87
| elucidation | voltage between the terminals of a cell or battery when being charged |
| subclassOf | CellVoltage |
ChemicalReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8a1c9a7b_978c_4421_a9c1_d0b07b42aab9
| elucidation | a process that results in the interconversion of chemical species |
| wikidataReference | https://www.wikidata.org/wiki/Q36534 |
| wikipediaReference | https://en.wikipedia.org/wiki/Chemical_reaction |
| iupacReference | https://doi.org/10.1351/goldbook.C01033 |
| restrictions |
|
| subclassOf | NaturalProcess |
| subclasses | Dissociation, GasEvolution, Dissolution, SideReaction, RedoxReaction |
ChromiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_510e4061_c4fa_49aa_a052_23ad56098eda
| elucidation | an electrode in which the primary active material consists of chromium or chromium compounds |
| subclassOf | ActiveElectrode |
CoatedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_92147e31_d015_4889_a092_04fbab033f15
| altLabel | Plate |
| elucidation | cell electrode consisting of a current collector and active material |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-02 |
| illustration |  |
| restrictions | |
| subclassOf | Electrode |
| subclasses | SingleCoatedElectrode, DoubleCoatedElectrode, PastedPlate |
Coating#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_09a7f560_9ddf_4c32_b067_b213eca5b0a1
| elucidation | a covering on the surface of an object |
| wikidataReference | https://www.wikidata.org/wiki/Q1570182 |
| wikipediaReference | https://en.wikipedia.org/wiki/Coating |
| restrictions |
|
| subclassOf | ElectrochemicalComponent |
| subclasses | ElectrodeCoating |
CoatingThickness#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3b938708_e7e4_4ac0_a959_9c04306302e7
| elucidation | thickness of the coating before any additional treatment is applied |
| subclassOf | Thickness, ElectrochemicalQuantity |
| subclasses | PositiveElectrodeCoatingThickness, CalenderedCoatingThickness, NegativeElectrodeCoatingThickness |
CobaltBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_838c115b_6bc9_4ce8_9f8d_86a6bf67742a
| elucidation | an electrode which contains mostly materials based on cobalt |
| subclassOf | ActiveElectrode |
| subclasses | LithiumCobaltOxideElectrode, SodiumCobaltOxideElectrode, SodiumCobaltPhosphateElectrode |
Note
often represented in IEC cell designations by the letter C
Note
this class is intended to enable designations based on IEC recommendations
CobaltBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7b811780_7251_481b_a4d3_97d437955099
| elucidation | an electrode in which the primary active material consists of cobalt or cobalt compounds |
| subclassOf | ActiveElectrode |
Coin#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2522cbd8_5382_457f_b9b5_775860f83357
| elucidation | a form factor describing a coin cell, which is a cylindrical cell where the height is less than the diameter |
| subclassOf | FormFactor |
CoinCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3979cd56_6256_414d_966c_7f723bf71e37
ConcentrationCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8a5083b0_cd23_4f8c_99e8_b9ccd6f9f3a2
| elucidation | electrochemical cell that has two half-cells separated by a wall permeable to ions, both containing the same electrolyte differing only in their ion concentrations |
| wikidataReference | https://www.wikidata.org/wiki/Q903563 |
| wikipediaReference | https://en.wikipedia.org/wiki/Concentration_cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-08 |
| illustration | 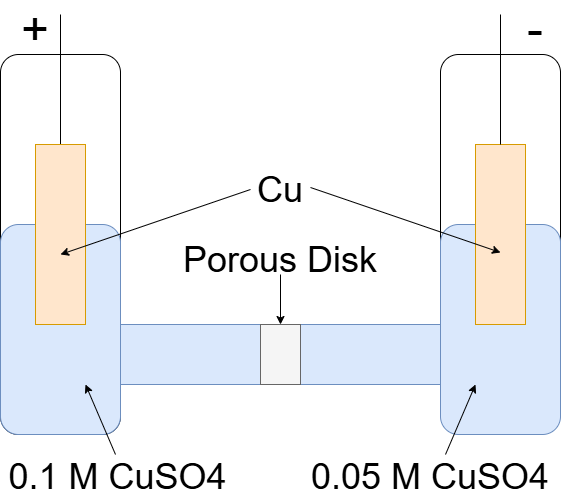 |
| subclassOf | ElectrochemicalDevice |
Note
a concentration cell produces a voltage as it attempts to reach equilibrium, which will occur when the concentration in both cells is equal.
ConcentrationLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5eae657f_5914_4252_85c6_3fc772dea113
| elucidation | limit on the amount concentration of a species in a phase, either imposed or naturally occurring |
| subclassOf | AmountConcentration, LimitQuantity |
| subclasses | MaximumConcentration, MiniumumConcentration |
ConcentrationOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9ed7210c_c4fa_467b_822d_ba12f885bdf4
| altLabel | ConcentrationPolarization; MassTransferOverpotential; MassTransferPolarization |
| elucidation | The concentration overpotential of an electrode reaction at a given electrode current density (c.d.) is basically the difference in equilibrium potentials across the diffusion layer. More precisely, it is the potential of a reference electrode (of the same electrode reaction as the working electrode ) with the interfacial concentrations which establish themselves at c.d., relative to the potential of a similar reference electrode with the concentrations of the bulk solution. From such a measured potential difference, with c.d. flowing, one needs to subtract the ohmic potential drop prevailing between the two electrodes. |
| wikipediaReference | https://en.wikipedia.org/wiki/Overpotential#Concentration_overpotential |
| iupacReference | https://goldbook.iupac.org/terms/view/C01230 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-08 |
| subclassOf | Overpotential |
ConductiveAdditive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_82fef384_8eec_4765_b707_5397054df594
| altLabel | ElectronicallyConductiveElectrodeAdditive |
| elucidation | a material added to an electrode for the purpose of increasing its electronic conductivity. |
| subclassOf | Additive |
Example
carbon black
ConductivityCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b525a629_a679_464f_bc5b_b49d2fc82686
| elucidation | a device used in electrochemistry to measure the resistance of a solution to determine its conductivity |
| subclassOf | ElectrochemicalDevice |
Note
Formed, in theory, by two 1 cm2 reversible electrodes spaced 1 cm apart, providing a uniform distribution of electrical field. In practice, however, a number of other configurations are used.
Connector#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d8a78cd2_8107_46dd_a198_0b64676efc00
| elucidation | conductor of electricity used for carrying current between components in an electric circuit |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-37 |
| subclassOf | Component |
Example
a connector electrically joins two cells, or a terminal of a cell to a terminal of the battery, or a terminal of the battery to an exterior circuit and also to auxiliary devices
ConstantCurrentCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_83f2b1e9_cb67_4dbf_977f_ba54bbae374f
| altLabel | GalvanostaticCharging |
| elucidation | charge during which the electric current is maintained at a constant value regardless of the battery voltage or temperature |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-38 |
| restrictions | |
| subclassOf | CurrentHold, Charging |
| subclasses | PulseCharging |
ConstantCurrentChargingCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_37c38b7e_9ded_481a_85fd_a467f1ee2b9f
| altLabel | ConstantCurrentChargeCapacity |
| elucidation | the capacity obtained during constant current charging of an electrochemical device |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| hiddenLabel | CC-Cap; CC-Cap/mAh |
| subclassOf | ChargingCapacity |
Note
Constant current charging capacity is often used to assess device performance and state-of-health. In a CC-CV charging process, if this value is significantly lower than the rated capacity under the same conditions, it may indicate underlying limitations. A decline over multiple cycles can be a sign of degradation.
ConstantCurrentConstantVoltageCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0cc8f231_0ce5_467e_9c76_29b2c80349ad
| altLabel | CCCV |
| elucidation | a two-step charging process in which a constant current is applied until a set upper cutoff voltage is reached and then the cell is potentiostatically held at that voltage until the current falls below some set threshhold value |
| restrictions | |
| subclassOf | TwoStepCharging |
ConstantCurrentConstantVoltageCycling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ac6c2371_995a_4fcb_89a2_15cb6615741e
| altLabel | CCCVCycling |
| elucidation | a cycling protocol in which an electrochemical cell is first put through a CCCV charging process followed by a constant current discharge process |
| restrictions | |
| subclassOf | Cycling |
ConstantCurrentDischarging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_53fe3f58_0802_41cf_af69_4784fc42cc30
| altLabel | GalvanostaticDischarging |
| elucidation | a discharging process in which the current is kept at a constant value |
| restrictions | |
| subclassOf | Discharging, CurrentHold |
| subclasses | PulseDischarging |
ConstantCurrentDischargingCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_08833ed2_6324_411a_b34b_fe64c44cd5ef
| altLabel | ConstantCurrentDischargeCapacity |
| elucidation | the capacity obtained during constant current discharging of an electrochemical device |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| hiddenLabel | DC-Cap; DC-Cap/mAh |
| subclassOf | DischargingCapacity |
ConstantPotentialPulses#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_84d37a37_88bd_47db_9425_31f73a81d38c
| elucidation | Signal consisting of successive pulses of electric potential of the same magnitude. |
| subclassOf | ElectricPotentialSignal |
ConstantPotentialSignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0d3e8340_4229_4fd3_b6dd_763bd566551d
| elucidation | signal consisting of a constant electric potential. |
| subclassOf | ElectricPotentialSignal |
ConstantPowerCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dcd4a15d_52cf_44fb_b826_df18e4baa89c
| elucidation | charge during which the electric power is maintained at a constant value |
| subclassOf | PowerHold |
ConstantPowerDischarging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a2f65954_1ed8_4faf_9efe_597018d03e8d
| elucidation | discharge during which the electric power is maintained at a constant value |
| subclassOf | PowerHold |
ConstantVoltageCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_acdeaa89_0e99_4941_8821_d5dea3d34912
| altLabel | ModifiedConstantVoltageCharging |
| elucidation | constant voltage charge where the electric current is limited to a predetermined value |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-50 |
| restrictions | |
| subclassOf | VoltageHold |
ConstantVoltageDischarging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9865e4f9_756d_4d94_a6fd_4102ab795f9e
| altLabel | PotentiostaticDischarging |
| elucidation | a discharging process in which the voltage between the terminals of the electrochemical cell is kept at a constant value |
| restrictions | |
| subclassOf | Discharging, VoltageHold |
ContinuousServiceTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_25d01d13_3ca5_4619_98c1_8ebbd01ad794
| elucidation | service test with an uninterrupted discharge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-48 |
| restrictions |
|
| subclassOf | Electrochemical |
ControlProperty#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_33e6986c_b35a_4cae_9a94_acb23248065c
| altLabel | ControlledQuantity; SetQuantity |
| elucidation | a target quantity in a control system |
| subclassOf | ObjectiveProperty |
| subclasses | InitialCondition, BoundaryCondition |
CopperBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_26b19a7c_59ca_4e1b_8fb9_ba061c22531e
| elucidation | an electrode in which the primary active material consists of copper or copper compounds |
| subclassOf | ActiveElectrode |
| subclasses | CopperOxideElectrode |
CopperOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a8edd38f_f8a5_41fb_9fc7_48f1866fd699
| altLabel | CupricOxideElectrode |
| elucidation | electrode in which the active material is cupric oxide (copper (II) oxide) |
| restrictions | |
| subclassOf | CopperBasedElectrode, MetalOxideElectrode |
Note
the combination with lithium metal and an organic electrolyte is designated using IEC electrochemical system letter code G
CoulombicEfficiency#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5696453c_9da7_41e2_bbda_603c1b90a8fc
| altLabel | CoulombEfficiency; CurrentEfficiency; FaradayEfficiency |
| elucidation | fraction of the electric current passing through an electrochemical cell which accomplishes the desired chemical reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-07 |
| hiddenLabel | CoulombEfficiency/% |
| subclassOf | RatioQuantity, ElectrochemicalPerformanceQuantity |
Coulometer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fb9bf7cb_dd4b_4391_99a1_628263dd6940
| altLabel | Voltameter |
| elucidation | measuring instrument to obtain the electrical charge passed in an experiment, or to produce a known amount of substance in a titration. |
| wikidataReference | https://www.wikidata.org/wiki/Q905539 |
| wikipediaReference | https://en.wikipedia.org/wiki/Voltameter |
| dbpediaReference | https://dbpedia.org/page/Voltmeter |
| subclassOf | MeasuringInstrument |
Note
Modern instruments for measuring trace water by Karl Fischer titration use the passage of a measured charge to produce iodine from iodide solution. They are known as Karl Fischer coulometers
Note
The silver coulometer is a primary reference measurement procedure [VIM 2.8] for charge and current. By weighing the mass of silver deposited in a known time at constant current, the charge and current are calculated from Faraday’s laws of electrolysis
CounterElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_871bc4a4_2d17_4b88_9b0f_7ab85f14afea
| altLabel | AuxiliaryElectrode |
| elucidation | electrode whose function is to carry the electric current flowing through the electrical circuit of an electrochemical cell, the electrochemical processes on its surface not being of interest |
| wikidataReference | https://www.wikidata.org/wiki/Q1768785 |
| wikipediaReference | https://en.wikipedia.org/wiki/Auxiliary_electrode |
| iupacReference | https://goldbook.iupac.org/terms/view/A00535 |
| subclassOf | Electrode |
Note
An auxiliary electrode is used in any three-electrode cell, together with a working electrode and reference electrode.
Note
An auxiliary electrode must not add an excessive potential burden to the cell. It is chosen for the efficiency of the electrochemical reaction and usually has a greater surface area than the working electrode.
CrystalizationOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8de9735b_374a_4a0f_b29f_71a50794cf94
| altLabel | CrystalizationPolarization |
| elucidation | part of the electrode polarization arising from crystal nucleation and growth phenomena |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-04 |
| subclassOf | ReactionOverpotential |
CumulativeCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_df9e9d49_12fa_4794_b482_e0dcd2a7bb66
| altLabel | TotalCapacity |
| elucidation | the total amount of electric charge that has cumulatively passed through an electrochemical device within a defined time interval, increasing monotonically over time |
| subclassOf | Capacity |
CurrentChangeLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_71f10616_15eb_4dc4_bc8d_ffaac3838af2
| elucidation | the limit on the value of the time change of the electric current |
| subclassOf | ElectrochemicalControlQuantity |
CurrentChangeRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6516ed26_9661_4d81_8c6d_15d2384d17c0
| elucidation | the first order time rate of change of electric current |
| novonixLabel | dIdt (V/h) |
| subclassOf | ElectrochemicalQuantity |
CurrentCollector#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_212af058_3bbb_419f_a9c6_90ba9ebb3706
| altLabel | ElectrodeCurrentCollector |
| elucidation | A good electron conductor support designed to transfer electrons from the external circuit to the active materials of the cell. |
| wikidataReference | https://www.wikidata.org/wiki/Q120907411 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=485-06-07 |
| subclassOf | ElectrochemicalComponent |
Example
Copper foil, Aluminum foil
CurrentCollectorTab#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6913368b_39cf_42da_ac51_c1b508930ac5
| elucidation | a part of the current collector that provides an interface between the electrode and the external electrical interface |
| illustration | 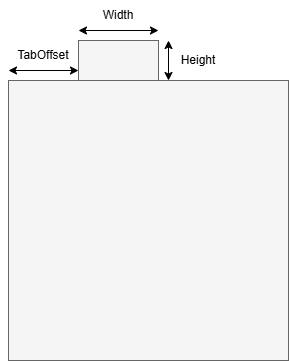 |
| restrictions |
|
| subclassOf | ElectrochemicalComponent |
CurrentControlledProcess#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5548f188_df00_4c05_ae98_7846e92efe36
| elucidation | a process in which the electric current is controlled |
| subclassOf | Process |
| subclasses | CurrentHold, StrippingChronopotentiometry, PotentiometricStrippingAnalysis, GalvanostaticCycling, CurrentPulsing |
CurrentDensityLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_76e7e556_f47e_47e2_b2ef_67aeed09c63e
| elucidation | a limit placed on the electric current density of an electrical system |
| subclassOf | ElectricCurrentDensity, ElectrochemicalPerformanceQuantity |
| subclasses | UpperCurrentDensityLimit, LowerCurrentDensityLimit |
CurrentHold#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_78a8f60b_10c2_41ee_9946_d35437e1edb5
| altLabel | GalvanostaticProcess |
| elucidation | a process in which the electric current is kept constant |
| restrictions | |
| subclassOf | CurrentControlledProcess |
| subclasses | ConstantCurrentDischarging, Resting, ConstantCurrentCharging |
CurrentLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_74de2c33_84fc_4c2f_afe1_56d169149114
| elucidation | limit on the electric current of an electrical system |
| subclassOf | ElectricCurrent, LimitQuantity |
| subclasses | LowerCurrentLimit, UpperCurrentLimit, MaximumPulseChargingCurrent, MaximumContinuousDischargingCurrent, MaximumContinuousChargingCurrent, MaximumPulseDischargingCurrent |
CurrentPotentialPlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b9a72491_8a50_4cac_a131_1e95d72b57ee
| altLabel | CurrentPotentialCurve; ElectricCurrentPotentialCurve |
| elucidation | plot of current versus applied potential (I-E) for a given system |
| subclassOf | ElectrochemicalPlot |
| subclasses | Voltammogram |
Note
The curve is sigmoidal in hydrodynamic voltammetry, voltammetry at microelectrodes and polarography, or peak-shaped in ac voltammetry, differential pulse voltammetry, square- wave voltammetry, stripping voltammetry, and derivative techniques.
CurrentPulseSignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_712c791a_d593_4732_af73_493f7bc50999
| elucidation | rapid, transient change in the amplitude of an electric current, from a baseline value to a higer or lower value, followed by a rapid return to the baseline value. |
| wikidataReference | https://www.wikidata.org/wiki/Q114979515 |
| subclassOf | ElectricCurrentSignal |
CurrentPulsing#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9f1ffb54_4403_4541_98c1_3a821c6d060f
| elucidation | the process of applying a current pulse to an electrochemical device |
| subclassOf | CurrentControlledProcess |
| subclasses | PulseCharging, PulseDischarging |
CurrentScanRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f046d602_22ea_4f9b_9101_319f510d39f0
| elucidation | The rate of change of an applied current with time. |
| subclassOf | CellCurrent, ElectrochemicalControlQuantity |
Note
Used in linear potentiometry and related techniques, where a linearly-changing current is imposed to a cell to measure its voltage response.
CurrentTimePlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b99cff7f_b13f_4075_aa88_62c04f8daacc
| altLabel | CurrentTimeCurve |
| elucidation | plot of the dependence of instantaneous current on time |
| subclassOf | ElectrochemicalPlot |
CycleIndex#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5766a819_c319_48ba_ab9a_ce3bd8fedee8
| altLabel | Cycle; CycleNumber |
| elucidation | the number of charge-discharge cycles that an electrochemical device has experienced |
| hiddenLabel | Cycle-Index; CycleNo |
| maccorLabel | cyc# |
| batteryArchiveLabel | Cycle_Index |
| newareLabel | Cycle ID |
| indigoLabel | cycle_count |
| arbinLabel | cycle_index |
| novonixLabel | Cycle Number |
| biologicLabel | cycle number |
| subclassOf | PureNumberQuantity, ElectrochemicalQuantity |
CycleLife#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ae782b14_88ce_4cdd_9418_12aca00be937
| altLabel | ElectrochemicalCycleLife |
| elucidation | the maximum number of cycles achieved in an electrochemical device before reaching some end-of-life criterion |
| subclassOf | PureNumberQuantity, ElectrochemicalPerformanceQuantity |
CycleNumberData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ca48d41c_f5ea_4bf8_84ce_2d67fd9dad98
| elucidation | cycle number data, usually resulting from an electrochemical measurement process |
| restrictions |
|
| subclassOf | Vector, PrimaryData |
CycleSeriesDataset#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d2f6f1a6_4dee_4c5e_9a69_32b9fe990d2f
| elucidation | a series of data points indexed in cycle order |
| restrictions | |
| subclassOf | Matrix |
Cycling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d3e2d213_d078_4b9a_8beb_62f063e57d69
| altLabel | ChargeCycle; ElectrochemicalCycling |
| elucidation | set of operations that is carried out on a secondary cell or battery and is repeated regularly in the same sequence. |
| wikidataReference | https://www.wikidata.org/wiki/Q5074261 |
| wikipediaReference | https://en.wikipedia.org/wiki/Charge_cycle |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-28 |
| restrictions | |
| subclassOf | Procedure |
| subclasses | PotentiostaticCycling, GalvanostaticCycling, FormationCycling, ConstantCurrentConstantVoltageCycling |
Note
in a secondary battery these operations may consist of a sequence of a discharge followed by a charge or a charge followed by a discharge under specified conditions
Note
this sequence may include rest periods
CyclingTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_435b22d4_c441_45ea_8c79_0cbec11fd287
| elucidation | a process that determines the number of complete charge-discharge cycles an electrochemical device can undergo before its capacity falls below a predefined threshold |
| restrictions | |
| subclassOf | ElectrochemicalTesting |
Cylindrical#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_780aeb7b_9eb2_4324_ba2d_1a874136b4e5
| elucidation | a form factor describing a cylindrical cell, which has the shape of a cylinder nominally described by a height and diameter |
| subclassOf | FormFactor |
CylindricalCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b1cf9fee_2164_4f95_8204_90f717373a8a
| elucidation | a round-type case with a height that is greater than or equal to the diameter |
| subclassOf | RoundCase |
| subclasses | electrochemistry.R32700, electrochemistry.R38136, electrochemistry.R54137, electrochemistry.R46800, electrochemistry.R21700, electrochemistry.R40920, electrochemistry.R18650, electrochemistry.R27660, electrochemistry.R38138, electrochemistry.R32134, electrochemistry.R26650, electrochemistry.R40108, electrochemistry.R19660, electrochemistry.R26700, electrochemistry.R54215 |
CylindricalCellElectrolyteFilling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dade3372_c229_47f2_b3c5_6ebb97a0f670
| elucidation | the process of filling an electrolyte into a cylindrical cell |
| bvco | https://bvco.ontology.link/BVCO_b1153b0b_1c2d_4890_aa1b_f9ed3ab0747a |
| subclassOf | ElectrolyteFilling |
CylindricalCellPackaging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_19a5cf32_2310_4ee8_a888_0d7bd8edc729
| elucidation | the process of inserting a dry jelly roll into a cylindrical cell container |
| subclassOf | CellPackaging |
D10ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b83c6435_6edf_470e_9725_538a853e08f7
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 10% of the sample's particles fall. |
| subclassOf | Diameter |
D15ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_92fc0dba_6f11_4019_a8cb_db2a5b1634f5
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 15% of the sample's particles fall. |
| subclassOf | Diameter |
D20ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_62e03250_8987_497b_85d5_1399aca9a0aa
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 20% of the sample's particles fall. |
| subclassOf | Diameter |
D25ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7f17f6bb_c1f9_4f4a_bfdf_d8974443d07b
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 25% of the sample's particles fall. |
| subclassOf | Diameter |
D30ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ed0362e_b7ae_482c_a7d0_2ca2eebda648
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 30% of the sample's particles fall. |
| subclassOf | Diameter |
D35ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c1c1288c_97c1_4f1f_8f42_6dfd34d028fd
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 35% of the sample's particles fall. |
| subclassOf | Diameter |
D40ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c8abaf09_300a_4008_a42a_2f4a3953ca7a
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 40% of the sample's particles fall. |
| subclassOf | Diameter |
D45ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9d60c4ab_d9ac_4dda_af0c_c64059326916
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 45% of the sample's particles fall. |
| subclassOf | Diameter |
D50ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3cfdfc10_a5cb_4e3e_b1a1_281010d1465c
| altLabel | MassMedianDiameter |
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 50% of the sample's particles fall. |
| subclassOf | Diameter, ElectrochemicalQuantity |
D55ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b095e977_dbc2_4a9e_9930_6af0dab089f1
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 55% of the sample's particles fall. |
| subclassOf | Diameter |
D5ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2e2d92f4_9fd5_4965_b15a_30b43c68e3e5
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 5% of the sample's particles fall. |
| subclassOf | Diameter |
D60ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8768c8f4_eaa8_4670_a3a0_91524e59a7f6
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 60% of the sample's particles fall. |
| subclassOf | Diameter |
D65ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1be95f7c_7381_4d60_a4fb_25b3db63be97
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 10% of the sample's particles fall. |
| subclassOf | Diameter |
D70ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e03f6800_92c5_4218_bb6d_1c919abaa063
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 10% of the sample's particles fall. |
| subclassOf | Diameter |
D75ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9cf8e627_a3d2_4f86_9bdc_e0e77996acde
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 10% of the sample's particles fall. |
| subclassOf | Diameter |
D80ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_97bc06e6_6141_47ec_9e81_fd9e6a5b07c7
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 10% of the sample's particles fall. |
| subclassOf | Diameter |
D85ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fbaa2d5e_b8f7_4a2f_9497_41c3698eb0ff
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 10% of the sample's particles fall. |
| subclassOf | Diameter |
D90ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8e943e12_ecc0_4093_899e_7226be6be7f2
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 90% of the sample's particles fall. |
| subclassOf | Diameter |
D95ParticleSize#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_02d2d1d1_241c_429b_b4e7_31f2c3dc4835
| elucidation | a statistical measure used in particle size distribution analysis, indicating the particle diameter below which 95% of the sample's particles fall. |
| subclassOf | Diameter |
Dendrite#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_34bc54e3_de4c_41f8_8d3a_d77c951ee23a
| elucidation | needle-like or tree-like formation of crystalline growth formed during deposition of a material |
| wikidataReference | https://www.wikidata.org/wiki/Q190136 |
| wikipediaReference | https://en.wikipedia.org/wiki/Dendrite_(crystal) |
| iupacReference | https://doi.org/10.1351/goldbook.D01588 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-07 |
| subclassOf | Role |
DepthOfDischarge#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1acab8af_9798_45df_a64b_a7030c57a4de
| altLabel | DoD |
| elucidation | the ratio of the discharged capacity to the rated capacity of an electrochemical device |
| wikipediaReference | https://en.wikipedia.org/wiki/Depth_of_discharge |
| subclassOf | RatioQuantity, ElectrochemicalQuantity |
DeviceDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ea21f71_d1bd_4714_a260_b991e6d4bcf7
| elucidation | the overall density of an electrochemical device, calculated as the quotient of the total mass and the total volume |
| bpxKey | ['Parameterisation','Cell','Density [kg.m-3]'] |
| cidemodKey | ['properties','density','value'] |
| subclassOf | Density |
DeviceLumpedSpecificHeatCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9cba2158_26ba_4dd7_b082_ba66dbb960c7
| elucidation | the lumped specific heat capacity of an electrochemical device |
| bpxKey | ['Parameterisation','Cell','Specific heat capacity [J.K-1.kg-1]'] |
| cidemodKey | ['properties','specific_heat','value'] |
| subclassOf | SpecificHeatCapacity |
DeviceSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ccde4e5f_ace4_41d1_b4d8_cbd63e6376e6
| elucidation | the external surface area of an electrochemical device |
| bpxKey | ['Parameterisation','Parameterisation','Cell','External surface area [m2]'] |
| cidemodKey | ['properties','external_surface_area','value'] |
| subclassOf | SurfaceArea |
DeviceTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_463950b9_fd8d_48de_a52d_b29cab026391
| elucidation | the temperature of an electrochemical device |
| batteryArchiveLabel | Cell_Temperature (C) |
| subclassOf | CelsiusTemperature |
DeviceVolume#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b4184e46_c53c_47cc_9bfc_186fd77836a5
| elucidation | the total volume of an electrochemical device, determined by its external dimensions |
| bpxKey | ['Parameterisation','Cell','Volume [m3]'] |
| cidemodKey | ['properties','volume','value'] |
| subclassOf | Volume |
DifferentialCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_10104499_6580_4867_9c5d_b9882c89c2f7
| altLabel | dQ/dV |
| elucidation | the derivative of the capacity of an electrochemical device with respect to the terminal voltage |
| newareLabel | dQ/dV(mAh/V) |
| subclassOf | ElectrochemicalQuantity |
DifferentialPotentialPulse#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b49e2355_392f_4e83_b630_7ff4581d767b
| elucidation | Small pulses of electric potential superimposed to a linear potential ramp or a staircase potential ramp. |
| subclassOf | ElectricPotentialSignal |
Note
The pulses are usually of constant height 10 to 100 mV and constant width 10 to 100 ms.
DiffuseLayerPotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e38f11d0_a16f_4fe8_8ec5_3fe4493b7759
| elucidation | electric potential difference between the rigid layer and the diffuse layer of a double layer |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-20 |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
DiffusionCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_17626b8e_dfce_4d3a_ae6c_5a7215d43a90
| elucidation | faradaic current that is controlled by the rate at which electroactive species diffuse toward (or away from) and electrode-solution interface. |
| wikidataReference | https://www.wikidata.org/wiki/Q1224527 |
| wikipediaReference | https://en.wikipedia.org/wiki/Diffusion_current |
| iupacReference | https://goldbook.iupac.org/terms/view/D01722 |
| subclassOf | FaradaicCurrent |
| subclasses | DiffusionLimitedCurrent |
DiffusionData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_50fef664_83c3_4ca5_808c_efaa1acc51b2
| elucidation | data that describes the mass diffusivity of some particle under some stated conditions |
| subclassOf | Dataset |
DiffusionLimitedCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5fb7a03f_d6dd_47ee_9317_0629681c7d00
| altLabel | LimitingDiffusionCurrent |
| elucidation | diffusion current of the potential-independent value that is approached as the rate of the charge-transfer process is increased by varying the applied potential, being greater than the rate of mass transport controlled by diffusion |
| iupacReference | https://goldbook.iupac.org/terms/view/L03534 |
| subclassOf | DiffusionCurrent |
Diffusivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_37b24a94_cae0_4d7a_9519_9f7692dec607
| elucidation | a measure of the rate at which particles or heat or fluids spread |
| subclassOf | ISQDerivedQuantity |
| subclasses | DiffusionCoefficient, GuestDiffusivityInPositiveElectrodeActiveMaterial, GuestDiffusivityInNegativeElectrodeActiveMaterial, ChargeCarrierDiffusivityInElectrolyte |
DirectCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_885b462e_f6bc_412d_8b94_9425e13af0c7
| altLabel | DC |
| elucidation | electric current that flows in a constant direction, i.e. a current with a constant sign |
| wikidataReference | https://www.wikidata.org/wiki/Q159241 |
| wikipediaReference | https://en.wikipedia.org/wiki/Direct_current |
| dbpediaReference | https://dbpedia.org/page/Direct_current |
| iupacReference | https://goldbook.iupac.org/terms/view/D01767 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
Note
I_{DC}
Note
The term ‘direct current’ should be used where there is ambiguity concerning the constancy of direction of current. See alternating current.
DirectCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_40ca9548_910a_48b6_9a26_f94095d349cd
| altLabel | ConstantCurrentSignal; DC |
| elucidation | Signal consisting of a constant electric current. |
| wikidataReference | https://www.wikidata.org/wiki/Q5163647 |
| wikipediaReference | https://en.wikipedia.org/wiki/Constant_current |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=131-11-22 |
| subclassOf | ElectricCurrentSignal |
Discharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_06d8e1ee_924a_4915_998d_33a69f41dadc
| altLabel | ElectrochemicalDischarging |
| elucidation | operation during which an electrochemical cell supplies electric energy as the result of chemical changes within the cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-23 |
| restrictions | |
| subclassOf | Procedure |
| subclasses | ConstantCurrentDischarging, ConstantVoltageDischarging |
Note
the opposite of a charging process
DischargingCRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_25e20915_c35d_4bee_ad31_736235a79780
| altLabel | DRate; DischargeRate |
| elucidation | [an indicator of the] electric current at which a battery is discharged |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-25 |
| subclassOf | CRate |
DischargingCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0141b5c2_9f15_46f4_82e6_92a104faa476
| altLabel | DischargeCapacity |
| elucidation | electric charge which an energy storage device can deliver under specified discharging conditions |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-14 |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| hiddenLabel | AH-OUT; CapD; CapD/mAh; Q discharge/mA.h |
| maccorLabel | wf dis cap |
| batteryArchiveLabel | Discharge_Capacity (Ah) |
| newareLabel | Capacitance_DChg(mAh) |
| arbinLabel | discharge_capacity |
| biologicLabel | Q discharge/mA.h |
| subclassOf | Capacity |
| subclasses | ConstantCurrentDischargingCapacity |
DischargingConstantCurrentPercentage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_457aed58_6e7e_42d5_9d78_b424c8d60aa3
| altLabel | ConstantCurrentDischargePercentage |
| elucidation | the percentage of the total discharge capacity that is obtained during a constant current discharge process |
| hiddenLabel | DC-Per; DC-Per/% |
| subclassOf | RatioQuantity |
DischargingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e4d666ee_d637_45cd_a904_dc33941ead4f
| elucidation | electric current delivered by a battery during its discharge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-24 |
| subclassOf | CellCurrent, ElectrochemicalQuantity |
DischargingData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a17b9fd0_b005_41eb_b685_e212fc4cecea
| elucidation | data that is obtained from experiment or simulation during a discharging process |
| subclassOf | Dataset |
DischargingEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ca36cbf3_1fed_4b88_9177_b4e16ad00cf7
| altLabel | DischargeEnergy |
| elucidation | energy delivered by a deviced under some specific discharge conditions |
| hiddenLabel | Energy discharge/W.h; EnergyD; EnergyD/mWh; WH-OUT |
| maccorLabel | wf dis e |
| batteryArchiveLabel | Discharge_Energy (Wh) |
| newareLabel | Engy_DChg(mWh) |
| arbinLabel | discharge_energy |
| biologicLabel | Energy discharge/W.h |
| subclassOf | StoredEnergy |
DischargingEnergyDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4f14e153_cffb_42bd_9a7f_ae40d51ad2cd
| elucidation | the energy density of a device obtained during a discharging process. |
| subclassOf | EnergyDensity |
DischargingPRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b6f33044_1892_41a4_ae89_8683d4225f8d
| elucidation | an indicator of the electric power at which an electrochemical device is discharged |
| subclassOf | PRate |
DischargingSpecificCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_884650fd_6cc6_4ec6_8264_c18fbe6b90ee
| altLabel | SpecificDischargeCapacity |
| elucidation | quotient of the capacity of a cell or battery [ or electrode or active material ] obtained during a discharge process by its mass. |
| hiddenLabel | SpeCapD; SpeCapD/mAh/g |
| subclassOf | SpecificCapacity |
DischargingSpecificEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9d57962c_e150_4e64_962b_8fd6a92c9234
| altLabel | SpecificEnergyDischarge |
| elucidation | the specific energy of an electrochemical device obtained during a discharge process |
| hiddenLabel | SpeEnergyD; SpeEnergyD/mWh/g |
| subclassOf | SpecificEnergy |
DischargingVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c7b26177_21bf_4787_b656_8e78edf27f88
| altLabel | DischargeVoltage; ClosedCircuitVoltage |
| elucidation | voltage between the terminals of a cell or battery when being discharged |
| source | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-28 |
| subclassOf | CellVoltage |
| subclasses | InitialDischargingVoltage |
Dissociation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b3b3868f_6edd_4ce8_a984_08f9cf391f90
| elucidation | process where molecules split up into ions due to being dissolved |
| wikidataReference | https://www.wikidata.org/wiki/Q189673 |
| wikipediaReference | https://en.wikipedia.org/wiki/Dissociation_(chemistry) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-01-08 |
| subclassOf | ChemicalReaction |
Dissolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_49263a32_eca6_4644_8144_0d3b14c26d0a
| elucidation | mixing of a substance into another substance with formation of one new homogeneous substance |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-01-04 |
| subclassOf | ChemicalReaction |
| subclasses | Electrodissolution, GasDissolution |
Note
the result of the dissolution of one or more solutes into a solvent is a solution
DoubleCoatedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_56f85b19_1384_4e88_b130_cb8e7984db83
| elucidation | an electrode that is coated on both sides of the current collector |
| illustration | 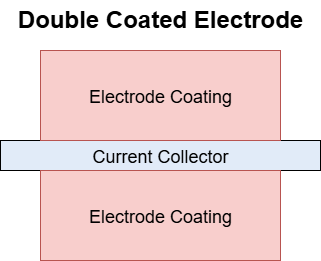 |
| subclassOf | CoatedElectrode |
DoubleLayer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2b50cdac_581f_48b9_87ca_bad5138ab58d
| altLabel | DL; EDL; ElectricalDoubleLayer |
| elucidation | a double layer of charges exists at the interface between two conducting media: One side carries a positive excess charge, which is balanced by a negative excess of equal magnitude on the other side. |
| wikidataReference | https://www.wikidata.org/wiki/Q904857 |
| wikipediaReference | https://en.wikipedia.org/wiki/Double_layer_(surface_science) |
| subclassOf | InterfacialRegion |
DoubleLayerCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a56fc557_9dea_42e6_b936_e9d62dcaf84f
| elucidation | non-faradaic current associated with the charging of the electrical double layer at the electrode-solution interface |
| iupacReference | https://goldbook.iupac.org/terms/view/D01847 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
DoubleLayerModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ac9723d1_bac0_4109_874e_32849de9299b
| altLabel | ElectricalDoubleLayerModel; ElectricalPolarizationLayerModel |
| elucidation | model representing the structure of an electrolyte at an electrode-electrolyte interface by a rigid layer formed by the charge carriers on the surface of the electrode and a diffuse layer formed by mobile ions in the electrolyte. |
| wikipediaReference | https://en.wikipedia.org/wiki/Double_layer_(surface_science) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-19 |
| subclassOf | Icon |
| subclasses | GouyChapmanModel, GrahameModel, BockrisDevanathanMuellerModel, HelmholtzModel, TrasattiBuzzancaModel, SternModel |
DropTimeInPolarography#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4b18c3f9_df10_4259_adb4_cd10842ff0be
| elucidation | the time that elapses between the instants at which two successive drops of liquid metal are detached from the tip of the capillary. |
| iupacReference | https://doi.org/10.1351/goldbook.D01862 |
| subclassOf | Duration, ElectrochemicalQuantity |
DroppingMercuryElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b76a778f_253f_4210_a67f_fb6444d0de26
| altLabel | DME |
| elucidation | mercury electrode formed by sequence of mercury drops falling from a small aperture |
| wikidataReference | https://www.wikidata.org/wiki/Q962292 |
| wikipediaReference | https://en.wikipedia.org/wiki/Dropping_mercury_electrode |
| subclassOf | MercuryElectrode |
Note
Other kinds of mercury drop electrodes are the static mercury drop electrode (SMDE), in which a drop is held at constant radius before being mechanically knocked off, and the hanging mercury drop elec- trode (HMDE), in which the whole experiment is performed on a single drop of mercury.
Note
The DME was introduced by Jaroslav Heyrovský (1890 – 1967). A drop is renewed every few seconds. Many metals amalgamate with mercury so that in the analysis of metal ions in aqueous solution the DME maintains a fresh surface throughout.
DryMixture#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0e5ef5a7_53c6_4d94_bffd_d0318ea0de6b
| elucidation | a mixture comprised of solid constituents |
| subclassOf | SolidMixture |
EdgeInsulator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bbc77932_643b_4603_a4e8_970ef06a76ad
| elucidation | part which insures insulation between the plate (electrode) edges and adjacent plates (electrodes) and the container side walls |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-19 |
| subclassOf | ElectrochemicalComponent |
EffectivePorousMediaQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_14d93129_d94d_42ff_a6f2_e8fca36ffec4
| elucidation | a physical quantity that is adjusted to consider the effects of porous media structure |
| subclassOf | CategorizedPhysicalQuantity |
| subclasses | EffectiveDiffusionCoefficient |
ElectricChargeSignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_276cfa84_3cc0_40c0_9f6a_57a3b776f47c
| elucidation | Time-dependent variation of electric charge passed through a system. |
| subclassOf | ElectricSignal |
ElectricCurrentData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4920de95_3a48_4669_b762_5a0b5232b52f
| elucidation | electric current data, usually resulting from an electrochemical measurement process |
| restrictions | |
| subclassOf | Vector, RawData |
ElectricCurrentMeasurement#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_158ae038_c64b_4cc0_aa56_650475490705
| elucidation | measurement of electric current |
| restrictions | |
| subclassOf | Measurement |
ElectricCurrentMeasurementResult#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7beef5fb_a406_4284_b85a_87d55cf46a0e
| elucidation | the result of an electric current measurement |
| subclassOf | MeasurementResult |
ElectricCurrentMeasuringSystem#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f693b744_930c_42ac_8e6f_627b22c6da3f
| elucidation | a system for measuing electric current |
| restrictions |
|
| subclassOf | MeasuringSystem |
Example
a digital coulometer connected to a computer
ElectricCurrentSignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_904b12e0_4a10_47b0_b7db_592aba215cb6
| elucidation | Time-dependent variation of electric current |
| subclassOf | ElectricSignal |
| subclasses | LinearCurrentRamp, AlternatingCurrent, DirectCurrent, CurrentPulseSignal, TriangularCurrentWaveform, StaircaseCurrentRamp |
ElectricPotentialMeasuringSystem#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3f9b2956_1465_4fe0_b0df_5e4784dac3b6
| elucidation | a system for measuring electric potential |
| restrictions | |
| subclassOf | MeasuringSystem |
Example
a digital voltmeter connected to a computer
ElectricPotentialSignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a03ce7e_d79f_412e_a103_b5d74de9f4d7
| elucidation | Time-dependent variation of electric potential |
| subclassOf | ElectricSignal |
| subclasses | SinusoidalPotentialWaveform, LinearPotentialRamp, PotentialPulse, IncreasingPotentialPulses, DifferentialPotentialPulse, ACVoltammetrySignal, SquareWavePotentialWaveform, SquareWaveVoltammetryWaveform, ConstantPotentialPulses, StaircasePotentialRamp, ConstantPotentialSignal, TriangularPotentialWaveform |
ElectricSignal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_898971cb_a9fc_4955_8abf_5d7163a9fe6c
| elucidation | Time dependent variation of an electric quantity. |
| subclassOf | Signal |
| subclasses | ElectricPotentialSignal, ElectricCurrentSignal, ElectricChargeSignal |
Electrocapillarity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5cb5548f_f774_4668_ad02_f0742581f2f1
| elucidation | change of the mechanical stress at the surface separating two bodies due to the presence of electric charges at the interface |
| wikidataReference | https://www.wikidata.org/wiki/Q9252431 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrocapillarity |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-02 |
| subclassOf | ElectrochemicalPhenomenon |
Electrocatalysis#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4d6f7f50_b343_4bd6_8612_2b8521a99c9b
| elucidation | increasing the rate of an electrode reaction by adding specific material to the electrode. |
| wikidataReference | https://www.wikidata.org/wiki/Q100318565 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-15 |
| restrictions | |
| subclassOf | ElectrochemicalPhenomenon |
Electrocatalyst#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a3b53904_22b1_42a9_a515_c8a3aed7e841
| elucidation | material that can cause electrocatalysis. |
| wikidataReference | https://www.wikidata.org/wiki/Q5357962 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrocatalyst |
| dbpediaReference | https://dbpedia.org/page/Electrocatalyst |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-16 |
| subclassOf | Catalyst |
Electrochemical#
IRI: https://w3id.org/emmo/domain/electrochemistry#Electrochemical
| elucidation | In electrochemical characterization, the measurement of potential, charge, or current is used to determine an analyte's concentration or to characterize an analyte's chemical reactivity |
| restrictions | |
| subclassOf | CharacterisationMeasurementProcess |
| subclasses | StorageTest, ContinuousServiceTest, CapacityTest, ReferencePerformanceTest |
ElectrochemicalCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6f2c88c9_5c04_4953_a298_032cc3ab9b77
| elucidation | system containing two electrodes that allow transport of electrons, separated by an electrolyte that allows movement of ions but blocks movement of electrons |
| wikidataReference | https://www.wikidata.org/wiki/Q80097 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrochemical_cell |
| dbpediaReference | http://dbpedia.org:8891/page/Electrochemical_cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-01 |
| illustration |  |
| restrictions |
|
| subclassOf | HolisticArrangement |
| subclasses | GalvanicCell, ElectrolyticCell, ThreeElectrodeElectrochemicalCell |
Note
composite system in which the supplied electric energy mainly produces chemical reactions or, conversely, in which the energy released by chemical reactions is mainly delivered by the system as electric energy
Note
It is important to distinguish between the conceptual electrochemical cell and the electrochemical device used in practice.
Tip
Model each electrode, electrolyte, and interface explicitly to represent charge transport correctly in simulations or ontological mappings.
Caution
Terminology varies across disciplines; in some contexts 'cell' may refer to the assembled device rather than the electrochemical unit of reaction.
Warning
Do not confuse the physical 'cell' with the manufactured 'device' that embeds it.
ElectrochemicalComponent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b5ea31c9_bb10_4276_980e_c3eceb1efc82
| elucidation | A component in an electrochemical system. |
| subclassOf | Component |
| subclasses | Electrolyte, SaltBridge, ActiveMaterial, ChargeCarrier, Additive, CurrentCollectorTab, CurrentCollector, Separator, Electrode, Particle, Case, Binder, Coating, CellLid, ActiveMaterialMix, CellCan, EdgeInsulator |
ElectrochemicalControlQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c5047d29_4e68_43ee_8355_3e8f05dc70a5
| elucidation | quantities that control electroanalytical techniques |
| subclassOf | ElectrochemicalQuantity |
| subclasses | StepSignalVoltage, AmplitudeOfAlternatingVoltage, PotentialScanRate, CurrentScanRate, PulseCurrent, BaselineCellVoltage, LimitQuantity, StepSignalCurrent, SamplingTime, LowerFrequencyLimit, TerminationQuantity, RestingTime, SignalReferencePotential, SamplingInterval, CurrentChangeLimit, AmplitudeOfAlternatingCurrent, PulseDuration, PulseVoltage, RotatingDiskSpeed, VoltageChangeLimit, UpperFrequencyLimit, StepDuration |
ElectrochemicalDegradationPhenomenon#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a0de817_addc_46a5_8ba2_255d48cdf366
| elucidation | A phenomenon that causes an electrochemical device to deviate from its ideal behaviour. |
| restrictions | |
| subclassOf | ElectrochemicalPhenomenon |
| subclasses | CapacityFade, ParticleCracking |
ElectrochemicalDevice#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0acd0fc2_1048_4604_8e90_bf4e84bd87df
| elucidation | a device whose primary function is facilitating the conversion between chemical and electrical energy. |
| illustration |  |
| restrictions |
|
| subclassOf | Device |
| subclasses | ConcentrationCell, FlowCell, Supercapacitor, ElectrolyticCapacitor, ConductivityCell, SealedCell, ThermogalvanicCell, HermeticallySealedCell, NonSpillableCell, VentedCell |
Note
an electrochemical device contains at least one electrochemical cell plus the apparati required to make it work as a practical device (e.g. terminals, container, etc.)
Note
The term refers to the engineered assembly; it must not be conflated with the electrochemical cell itself.
Tip
When documenting a device, identify the number and arrangement of cells and the presence of auxiliary components such as separators, membranes, and power electronics.
Important
Electrochemical devices typically include safety and control subsystems that are not part of the electrochemical cell (e.g. pressure relief, BMS, valves, current collectors).
ElectrochemicalDeviceAging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5076e90b_ed93_4845_87b1_e26bc8a4f0fe
| elucidation | the process of aging an electrochemical device as part of the manufacturing process |
| subclassOf | IntentionalAgency |
ElectrochemicalDeviceAssembly#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f2a034b6_401d_46bf_a258_cca87932c7af
| elucidation | the process of assembling components into an electrochemical device |
| subclassOf | IntentionalAgency |
ElectrochemicalDeviceFinishing#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_acd4bba1_e0f2_4114_b8f8_963645c5dac5
| elucidation | the process of preparing an electrochemical device for its intended functional performance |
| subclassOf | IntentionalAgency |
ElectrochemicalDeviceManufacturing#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cd5a6260_5d89_4a14_8e19_5f64742b64ac
| elucidation | the process of manufacturing an electrochemical device |
| subclassOf | IntentionalAgency |
ElectrochemicalDoubleLayerCapacitor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b91180e7_97ae_49e2_bf82_5bf720e7fa66
| altLabel | DoubleLayerCapacitor; EDLC; ElectrochemicalCapacitor |
| elucidation | Device that stores electrical energy using a double layer in an electrochemical cell. |
| subclassOf | Supercapacitor |
Note
stores energy electrostatically in the Helmholtz Layer
ElectrochemicalHalfCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9da958fc_f76d_4654_8a78_99b5f98c118c
| altLabel | HalfCell |
| elucidation | a system comprising one electrode in contact with an ionic conductor. |
| wikidataReference | https://www.wikidata.org/wiki/Q903509 |
| wikipediaReference | https://en.wikipedia.org/wiki/Half-cell |
| restrictions | |
| subclassOf | HolisticArrangement |
Note
Do not conflate a half-cell (a physical arrangement) with a half-reaction (a formal redox equation component).
Note
A half-cell becomes operational when paired with at least one other half-cell to form an electrochemical cell; the pair allows charge balance through ionic and electronic pathways.
Warning
Do not conflate an ElectrochemicalHalfCell (the combination of one electrode and one electrolyte) with a half-cell device, which is an experimental setup used to measure the properties of an electrode relative to a reference electrode.
Important
A single half-cell cannot sustain continuous current by itself; closed-circuit operation requires a counter half-cell and an external electronic path.
ElectrochemicalImmunity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4bc38e99_1978_4143_a766_fbb57f2cf46e
| elucidation | situation in which a material in a given environment undergoes negligible electrochemical corrosion. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-03 |
| subclassOf | ElectrochemicalPhenomenon |
| subclasses | CathodicProtection |
Example
cathodic protection
ElectrochemicalImpedanceSpectroscopyData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c052cab7_46eb_4a87_b7a4_05be2572db22
| altLabel | EISData |
| elucidation | a dataset resulting from an electrochemical impedance spectroscopy experiment or simulation |
| subclassOf | Dataset |
ElectrochemicalInterface#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_50044b99_b858_433b_a32d_23d1e1cf88b2
| elucidation | the two-dimensional space comprising the boundary between two electrochemical constituents |
| subclassOf | Interface |
| subclasses | ElectrodeElectrolyteInterface, LiquidJunction |
Note
The area where electrochemical reactions normally take place.
ElectrochemicalKineticQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_21745019_2830_4395_bca7_15ddfd266673
| elucidation | a quantity that relates to the kinetics of a reaction. |
| subclassOf | ElectrochemicalQuantity |
| subclasses | ExchangeCurrentDensity, ExchangeCurrent, NumberOfElectronsTransferred, ChargeTransferCoefficient, ReactionRate, ReactionRateConstant |
ElectrochemicalMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ebdb68e9_c4b5_4d57_a042_c0f51d446755
| elucidation | a material that takes active part in an electrochemical reaction |
| subclassOf | emmo.EMMO_68390bfb_e307_479d_8f78_d66d8773cb1d |
| subclasses | Electrolyte, ActiveMaterial |
ElectrochemicalMeasurementProcess#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f4ce4df2_d7e6_470f_8eab_3a911adaaf0f
| elucidation | The measurement step in an electrochemical testing procedure or characterization method with output data for voltage, current, time, and temperature. |
| restrictions | |
| subclassOf | CharacterisationMeasurementProcess |
ElectrochemicalMethod#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f49b84d4_e1f9_424c_bb22_8cea23c0a7d4
| elucidation | a method used in electrochemistry |
| subclassOf | IntentionalAgency |
ElectrochemicalMigration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_63ea1c9b_0bda_4a69_9745_efb08e6be685
| elucidation | transport of ions in an electrolyte due to an electric field |
| wikidataReference | https://www.wikidata.org/wiki/Q30587730 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrochemical_migration |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-06 |
| subclassOf | ElectrochemicalPhenomenon |
ElectrochemicalPerformanceQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_02eb0465_4f94_453c_8c1f_5ddf80134634
| elucidation | a quantity that indicates the performance of an electrochemical device |
| subclassOf | ElectrochemicalQuantity |
| subclasses | SpecificPower, StoredEnergy, EnergyDensity, AreicCapacity, SpecificCapacity, ChargeEfficiency, InternalResistance, electrochemistry.PowerDensity, SpecificEnergy, EnergyEfficiency, ShelfLife, CycleLife, CoulombicEfficiency, CurrentDensityLimit |
ElectrochemicalPhenomenon#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_19abaccd_43be_4048_965c_e4fb63c5951b
| elucidation | a chemical phenomenon that is accompanied by the flow of electric current |
| subclassOf | NaturalProcess |
| subclasses | Electrolysis, Leakage, ElectrodePolarisation, ElectrochemicalImmunity, Electrocatalysis, InterphaseGrowth, ElectrodePassivation, GassingOfACell, Electroosmosis, ElectrochemicalMigration, ElectrochemicalReaction, Electrocapillarity, ElectrolyteCreep, ElectrochemicalDegradationPhenomenon, ThermalRunaway, Electrochemiluminescence |
ElectrochemicalPlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ddade648_2033_47b6_bc36_b562a9af591e
| elucidation | Graphical representation of a dataset resulting from the measurement of the response of an electrochemical system. |
| subclassOf | Representation |
| subclasses | BodePlot, NyquistPlot, PotentialTimePlot, CurrentTimePlot, ChargeTimePlot, CurrentPotentialPlot |
Note
Shows the relationship between two or more electrochemical variables.
ElectrochemicalPotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1422cde1_929e_46b6_b0dc_1010eebc5dfd
| elucidation | the chemical potential of an ion in the presence of an electric potential |
| wikidataReference | https://www.wikidata.org/wiki/Q62525 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrochemical_potential |
| iupacReference | https://goldbook.iupac.org/terms/view/E01945 |
| restrictions | |
| subclassOf | ISQDerivedQuantity, ElectrochemicalThermodynamicQuantity |
ElectrochemicalProperty#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_900e357f_2ee3_425a_a0b6_322661117787
| elucidation | a property that is typical in the electrochemistry domain |
| subclassOf | NominalProperty |
| subclasses | Polarity, ChargeRetention, Prismatic, ElectrolyteContainment |
ElectrochemicalPseudocapacitor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ca0527c1_f682_4eea_aca5_f3ae66a9ddce
| elucidation | a type of supercapacitor that uses metal oxide or conducting polymer electrodes with a high amount of electrochemical pseudocapacitance additional to the double-layer capacitance |
| wikidataReference | https://www.wikidata.org/wiki/Q7254569 |
| wikipediaReference | https://en.wikipedia.org/wiki/Pseudocapacitor |
| subclassOf | Supercapacitor |
Note
stores energy electrochemically via Faradaic mechanisms
ElectrochemicalQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_aecc6094_c6a5_4a36_a825_8a497a2ae112
ElectrochemicalReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a6a69e90_06b5_45b1_83cf_7c0bf39b2914
| altLabel | ChargeTransferReaction |
| elucidation | a chemical reaction in an electrolyte involving a transfer of electrons between chemical components or between chemical components and an electrode |
| wikidataReference | https://www.wikidata.org/wiki/Q120892494 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-01; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-01 |
| subclassOf | ElectrochemicalPhenomenon, RedoxReaction |
| subclasses | ElectrodeReaction |
Note
electrochemical reactions occur at electrochemical interfaces
ElectrochemicalRelation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3d805c2a_4801_440e_9e4d_0fa5585c76ae
| elucidation | A material relation in electrochemistry. |
| subclassOf | MaterialRelation |
| subclasses | NernstEquation, NernstEinsteinEquation, ButlerVolmerEquation |
ElectrochemicalStabilityLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8f4b90ef_fea4_47c9_99f5_a9b3290a505d
| elucidation | electric potential at which a material undergoes an oxidation or reduction decomposition |
| subclassOf | ElectricPotential, ElectrochemicalThermodynamicQuantity |
ElectrochemicalTestingProcedure#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_830d02dd_f897_4c3c_95a7_c5e5eafa346a
| elucidation | the process of evaluating the performance and operational capabilities of an electrochemical device under specific conditions to determine its practical suitability for intended applications |
| subclassOf | Procedure |
ElectrochemicalThermodynamicQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2d896559_eee3_447c_9759_87c854a4266a
| elucidation | an electrochemical quantity describing concepts from thermodynamics |
| subclassOf | ElectrochemicalQuantity |
| subclasses | MembranePotential, ElectrochemicalPotential, MolarElectrochemicalPotential, ElectrochemicalStabilityLimit, OpenCircuitVoltage, ElectrochemicalWindow, IonConcentration |
ElectrochemicalTransportQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4a450a27_b84a_4c70_a3a9_15ec30e2f30b
| elucidation | an electrochemical quantity related to the transport of mass, charge, or energy. |
| subclassOf | ElectrochemicalQuantity |
| subclasses | MigrationCurrent, IonicConductivity, InternalConductance, LimitingMolarConductivity |
ElectrochemicalWindow#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_129926b6_fc30_441d_b359_29b44c988514
| altLabel | ElectrochemicalStabilityWindow |
| elucidation | The electrode electric potential range between which the substance is neither oxidized nor reduced. |
| wikidataReference | https://www.wikidata.org/wiki/Q759643 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrochemical_window |
| subclassOf | ElectricPotential, ElectrochemicalThermodynamicQuantity |
ElectrochemicallyActiveSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bad1b6f4_1b26_40e2_b552_6d53873e3973
| altLabel | EASA; ECSA; ElectrochemicalActiveSurfaceArea |
| elucidation | the area of the electrode material that is accessible to the electrolyte that is used for charge transfer and/or storage |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=485-02-09 |
| subclassOf | ElectrodeSurfaceArea |
Electrochemiluminescence#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_96309fa9_e157_48fe_9fda_41003860a3c0
| altLabel | ECL; ElectrogeneratedChemiluminescence |
| elucidation | chemiluminescence produced in electrode reactions |
| wikidataReference | https://www.wikidata.org/wiki/Q755961 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrochemiluminescence |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-18 |
| subclassOf | ElectrochemicalPhenomenon |
Electrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0f007072_a8dd_4798_b865_1bf9363be627
| elucidation | electronically conductive part in electric contact with a medium of lower electronic conductivity and intended to perform one or more of the functions of emitting charge carriers to or receiving charge carriers from that medium or to establish an electric field in that medium |
| etymology | From Greek elektron (‘amber, electricity’) + hodos (‘way’): ‘the way of electricity’. Proposed to replace vague ‘poles/plates’ with a neutral, process-descriptive term. Introduced by Michael Faraday, with support from William Whewell and Whitlock Nicholl. |
| wikidataReference | https://www.wikidata.org/wiki/Q176140 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrode |
| dbpediaReference | https://dbpedia.org/page/Electrode |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-21; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-03 |
| restrictions | |
| subclassOf | ElectrochemicalComponent |
| subclasses | Cathode, NegativeElectrode, CoatedElectrode, InertElectrode, WorkingElectrode, NonPolarizableElectrode, ActiveElectrode, RectangularElectrode, PorousElectrode, PolarizableElectrode, PositiveElectrode, RoundElectrode, CounterElectrode, CarbonBasedElectrode, Anode, IndicatorElectrode |
Note
Alternative definition: Half-cell consisting of at least one electron conductor and at least one ionic conductor (electrolyte). - Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019)
Note
Electron conductor in an electrochemical cell connected to the external circuit.
Note
In some electroanalytical techniques (conductometry and differential potentiometry), two indicator electrodes are used. For many analytical measurements in cells with current flow, a three-electrode cell is used.
Note
Many types of electrodes are used. These may be classified into groups according to their composition, form and size, and according to their operating mode. See section 4.
Note
The current flow through electrochemical cells may be zero or non-zero. An electrochemical cell with current flow can operate either as a galvanic cell or an electrolytic cell.
ElectrodeCalendering#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_38a2b407_3be9_4b66_86cf_b961cdffb4c9
| elucidation | the process of calendering an electrode |
| subclassOf | IntentionalAgency |
ElectrodeCoating#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cfcf6ca6_70e9_4369_9314_7f20b3efbf21
| elucidation | the process of applying a coating to a substrate to manufacture an electrode |
| subclassOf | IntentionalAgency |
ElectrodeCoating#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_403c300e_09b9_400b_943b_04e82a3cfb56
| elucidation | a coating layer that is part of an electrode and contains one or more active chemical substances together with auxiliary components |
| restrictions | |
| subclassOf | Coating |
Example
A LiFePO4 cathode coating comprising LFP powder (active), PVDF (binder), and carbon black (conductive additive)
ElectrodeCoverageDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_63a8f9d2_0cf6_484d_b996_2e5c3f0a3c73
| altLabel | ElectrodeCoverage |
| elucidation | amount of a chemical modifier at an electrode surface per unit area |
| restrictions | |
| subclassOf | ISQDerivedQuantity, ElectrochemicalQuantity |
ElectrodeDrying#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_36c2e19c_cb1a_41e9_bcd5_baf9dfd21905
| elucidation | the process of drying an electrode |
| subclassOf | Drying |
ElectrodeElectrolyteInterface#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9c73aff8_1c82_4116_a6be_78e21982b69d
| altLabel | ActiveSurfaceOfAnElectrode |
| elucidation | interface between an electrolyte and an electrode where an electrode reaction takes place. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-05; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-26 |
| subclassOf | ElectrochemicalInterface |
ElectrodeGeometricSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fa7790d6_07bb_4b0f_9965_55966828f5f3
| elucidation | the interfacial area, determined on the assumption that the interface is truly flat (2-dimensional) and calculated using the geometric data of the involved surfaces |
| subclassOf | ElectrodeSurfaceArea |
ElectrodeManufacturing#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_aaeb3c53_3199_4851_95d1_9cf1503a1b4b
| elucidation | the process of manufacturing and electrode |
| subclassOf | IntentionalAgency |
ElectrodeNotching#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b50d66be_092e_4aca_b8fd_59aa30e47d7c
| elucidation | the process of notching tabs into an electrode sheet |
| subclassOf | IntentionalAgency |
ElectrodePair#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d907805f_678b_4ab6_8b56_59631684f84b
| altLabel | PlatePair |
| elucidation | assembly of one positive plate, one negative plate and the associated separator if any |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-09 |
| subclassOf | Assembled |
ElectrodePassivation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_01260656_ac32_472e_9513_a607366538ec
| altLabel | Passivation |
| elucidation | formation of compounds that reduces the conductivity at the surface of an electrode |
| wikidataReference | https://www.wikidata.org/wiki/Q917260 |
| wikipediaReference | https://en.wikipedia.org/wiki/Passivation_(chemistry) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-16 |
| restrictions | |
| subclassOf | ElectrochemicalPhenomenon |
ElectrodePolarisation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2e6933aa_4522_4f16_a437_37110e6cbd0d
| altLabel | ElectrodePolarization; Polarisation; Polarization |
| elucidation | accumulation or depletion of electric charges at an electrode, resulting in a difference between the electrode potential with current flow, and the potential without current flow or equilibrium electrode potential |
| wikidataReference | https://www.wikidata.org/wiki/Q2698605 |
| wikipediaReference | https://en.wikipedia.org/wiki/Polarization_(electrochemistry) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-15 |
| restrictions |
|
| subclassOf | ElectrochemicalPhenomenon |
| subclasses | CathodicPolarisation, AnodicPolarisation |
ElectrodePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f509645f_eb27_470e_9112_7ab828ed40d3
| elucidation | electric potential at an electrode, reported as the difference in potential relative to a reference electrode |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrode_potential |
| iupacReference | https://goldbook.iupac.org/terms/view/E01956 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-11 |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
| subclasses | EquilibriumElectrodePotential |
ElectrodeReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2e3e14f9_4cb8_45b2_908e_47eec893dec8
| elucidation | electrochemical reaction involving the transfer of electrons between electrolyte and electrode |
| iupacReference | https://doi.org/10.1351/goldbook.E01960 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-04 |
| subclassOf | ElectrochemicalReaction |
| subclasses | CathodicReaction, AnodicReaction |
Note
An interfacial reaction that necessarily involves a charge-transfer step.
Note
The electrode (or interfacial) reaction involves all the processes (chemical reaction, structural reorganization, adsorption) accompanying the charge transfer step.
ElectrodeRealSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a82e16c3_b766_482f_be94_b8e9af37f6fc
| elucidation | surface area of an electrode that takes into account non-idealities of the interface (roughness, porosity, etc.) and can be measured by a variety of electrochemical methods |
| subclassOf | ElectrodeSurfaceArea |
ElectrodeSeparation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eeafeec2_2969_4886_b572_07ce39d3ee63
| elucidation | the process of separating individual electrodes from an electrode sheet |
| subclassOf | IntentionalAgency |
ElectrodeSlitting#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5096e449_4d92_420e_ae6d_0e156f6e4ebd
| elucidation | the process of slitting an electrode into smaller sections |
| subclassOf | IntentionalAgency |
ElectrodeStack#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_27662d03_1bcf_4393_a239_32e31b760839
| altLabel | PlatePack |
| elucidation | finished assembly of the positive and negative plate (electrode) groups with interleaved separators and terminals or intercell connectors |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-08 |
| subclassOf | Assembled |
ElectrodeStacking#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a490e278_8c6d_4a89_ad0e_ffa48366cd75
| elucidation | the process of stacking layers of negative electrode, separator, and positive electrode to create a dry stack |
| subclassOf | IntentionalAgency |
ElectrodeSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_47ab1dad_cc09_4fd8_af23_acb36fb680dd
| elucidation | area of electrode - solution interface. |
| subclassOf | SurfaceArea, ElectrochemicalQuantity |
| subclasses | ElectrodeRealSurfaceArea, ElectrodeGeometricSurfaceArea, ElectrochemicallyActiveSurfaceArea |
ElectrodeWinding#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_707f001f_d2b9_46c2_ac1b_9cd039bcf00f
| elucidation | the process of forming a negative electrode, separator, and positive electrode into a wound dry jelly roll |
| subclassOf | IntentionalAgency |
Electrodeposition#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f0c24970_4c14_4207_bd78_5f2181a67085
| elucidation | the process of forming a film or a bulk material using an electrochemical process where the electrons are supplied by an external power supply |
| subclassOf | IntentionalAgency, CathodicReaction |
| subclasses | Electroplating |
Note
Used in manufacturing, coating, and electrorefining applications.
Example
Deposition of metallic zinc from Zn^{2+} ions in an electroplating bath.
Electrodissolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4df84ec1_8a1a_4770_963f_bf48009bd043
| elucidation | the electrochemical dissolution of a material to soluble species |
| subclassOf | Dissolution, AnodicReaction |
Note
It can be regarded as the reversal of electrodeposition.
Example
Dissolution of metallic copper as Zn^{2+} during corrosion or anodization.
Electrolyser#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a6232fa7_ec1e_4529_bb9f_3598d3d0b106
| altLabel | Electrolyzer |
| elucidation | a device that performs electrolysis. |
| wikidataReference | https://www.wikidata.org/wiki/Q125141087 |
| subclassOf | FlowCell |
Electrolysis#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e2a1dae1_05e4_4bd1_a39d_0eb10db482bc
| elucidation | method of separating and neutralizing ions by an electric current in an electrolytic cell |
| etymology | From Greek elektron (‘electric’) + lysis (‘loosening, dissolution’): ‘decomposition by electricity’. Part of Faraday’s systematic terminology built to avoid theoretical commitments. Coined by Michael Faraday with support from William Whewell and Whitlock Nicholl. |
| wikidataReference | https://www.wikidata.org/wiki/Q64403 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrolysis |
| dbpediaReference | https://dbpedia.org/page/Electrolysis |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-09 |
| subclassOf | ElectrochemicalPhenomenon |
Electrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fb0d9eef_92af_4628_8814_e065ca255d59
| elucidation | a material in which the mobile species are ions and free movement of electrons is blocked. |
| etymology | From Greek elektron ('electric') + lytēs (, ‘looser’): ‘that which is set free or made to pass by electricity’; adopted to denote the conducting medium undergoing electrolysis. Coined by Michael Faraday with support from William Whewell and Whitlock Nicholl. |
| wikidataReference | https://www.wikidata.org/wiki/Q162908 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrolyte |
| dbpediaReference | https://dbpedia.org/page/Electrolyte |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-01-02; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-29 |
| restrictions | |
| subclassOf | ElectrochemicalComponent, ElectrochemicalMaterial |
| subclasses | Catholyte, LiquidElectrolyte, SolidElectrolyte, Anolyte, PolymerElectrolyte, BinaryElectrolyte |
Note
A solid electrolyte is a solid material where the predominant charge carriers are ions. For example: NASICON (Na Super Ionic Conductor), which has the general formula Na1+xZr2P3-xSix O12 , 0 < x < 3.
Note
An ionic liquid is an electrolyte composed of a salt that is liquid below 100 °C. Ionic liquids have found uses in electrochemical analysis, because their unconventional properties include a negligible vapor pressure, a high thermal and electrochemical stability, and exceptional dissolution properties for both organic and inorganic chemical species.
Note
conducting medium in which the flow of electric current is accompanied by the movement of ions
Note
liquid or solid substance containing mobile ions that render it conductive
Note
substance that provides ions on dissolution in a solvent or on melting
ElectrolyteAdditive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_554286d4_1f46_44c0_bebc_4ddae00dbce1
| elucidation | A substance added to an electrolyte to alter its properties |
| subclassOf | Additive |
ElectrolyteContainment#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_83928dce_9746_4452_a9f9_da4366a20ca4
| elucidation | ability of a cell or battery to contain its electrolyte under specified mechanical and environmental conditions |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-31 |
| subclassOf | ElectrochemicalProperty |
ElectrolyteCreep#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3f6c9e09_5f23_41cc_9f85_7de365cef089
| elucidation | gradual and slow spreading of an electrolyte film on the external surface of a cell or battery |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-30 |
| subclassOf | ElectrochemicalPhenomenon |
Note
electrolyte creep is sometimes indicated by the presence of a visible solid deposit or wet spots
ElectrolyteFilling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ff7f5ab4_d0c0_4455_9bcc_3d80346e90ee
| elucidation | the insertion of liquid electrolyte into an electrochemical device |
| subclassOf | IntentionalAgency |
| subclasses | PrismaticCellElectrolyteFilling, PouchCellElectrolyteFilling, CylindricalCellElectrolyteFilling |
ElectrolyteLevelIndicator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c05692eb_ae92_4e03_b355_f259f9089cb8
| elucidation | device used to assist in the measurement of the level of electrolyte in a cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-52 |
| subclassOf | Device |
ElectrolyteManufacturing#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b85ca559_eeef_4763_ac2e_7e658bf64b45
| elucidation | the process of manufacturing electrolyte |
| subclassOf | IntentionalAgency |
ElectrolyteSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fa22874b_76a9_4043_8b8f_6086c88746de
| altLabel | ElectrolyticSolution |
| elucidation | a liquid electrolyte that consists of solutes dissolved in a solvent |
| restrictions |
|
| subclassOf | LiquidSolution, LiquidElectrolyte |
| subclasses | SupportingElectrolyte, AqueousElectrolyte, OrganicElectrolyte |
ElectrolyticCapacitor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_900d95fb_863d_4142_a96d_369fb39e2639
| elucidation | capacitor consisting of a metallic plate as first conductor, a very thin oxide film formed on the metal as the dielectric and an electrolyte as second conductor |
| wikidataReference | https://www.wikidata.org/wiki/Q1326992 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrolytic_capacitor |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-04 |
| subclassOf | ElectrochemicalDevice |
ElectrolyticCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e931087f_7681_4096_b200_5223bcc47eb4
| elucidation | electrochemical cell intended to produce chemical reactions |
| wikidataReference | https://www.wikidata.org/wiki/Q2608426 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electrolytic_cell |
| dbpediaReference | https://dbpedia.org/page/Electrolytic_cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-06 |
| subclassOf | ElectrochemicalCell |
| subclasses | PhotoelectrolyticCell |
Note
An electrochemical cell that requires input of work to drive the reaction.
Note
Electrochemical cell in which electrical energy is converted into chemical energy.
ElectrolyticConductivityData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_571c1ae9_c4bf_48ab_babf_536153d22a0b
| elucidation | data that describes the electrolytic conductivitysome material under some stated conditions |
| subclassOf | Dataset |
ElectronicConductivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ce74d2dc_d496_4116_b2fb_3e83d88bc744
| elucidation | measure of a substance's tendency towards electronic conduction |
| subclassOf | ElectricConductivity, ElectrochemicalQuantity |
| subclasses | NegativeElectrodeElectronicConductivity, PositiveElectrodeElectronicConductivity |
Electroosmosis#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5641910f_6e69_4ce4_be84_4b1bf14b8916
| elucidation | movement of a fluid through a diaphragm, produced by application of an electric field |
| wikidataReference | https://www.wikidata.org/wiki/Q241065 |
| wikipediaReference | https://en.wikipedia.org/wiki/Electro-osmosis |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-01 |
| subclassOf | ElectrochemicalPhenomenon |
Electroplating#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a2b92d2e_4431_411e_8da5_a4c08bac2c0e
| altLabel | Galvanizing |
| elucidation | process inside an electrolytic cell used to coat a conductive object with a layer of a material |
| wikipediaReference | https://en.wikipedia.org/wiki/Electroplating |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-17 |
| subclassOf | Electrodeposition |
Note
The process of depositing a usually thin layer of metal upon a usually metallic substrate (or any other conductor, e.g. graphite), in order to improve the appearance, and/or to change the surface properties of the substrate.
ElectrospunMesh#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9e2f4509_e3f1_4bff_a5f4_8792c372e338
| elucidation | a nonwoven mesh made by electrospinning, in which an electric field draws polymer jets into ultra-thin fibers that form a porous, interconnected network with high surface area with tunable porosity and fiber diameter. |
| subclassOf | ManufacturedMaterial |
EnergyCalculation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cbb95634_450c_4332_980c_d37baadd7619
| elucidation | data processing procedure that determines the energy based on time data, voltage data, and electric current data |
| restrictions |
|
| subclassOf | MeasurementDataPostProcessing |
EnergyDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4aa1b96e_44a0_4b1a_a0ac_723d0223d80b
| altLabel | EnergyDensityOfStorage; VolumetricEnergyDensity |
| elucidation | the quotient of the energy of an energy-storage device or system and its volume |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-22 |
| restrictions | |
| subclassOf | Intensive, ElectrochemicalPerformanceQuantity |
| subclasses | ChargingEnergyDensity, DischargingEnergyDensity |
EnergyEfficiency#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_79198264_cdf5_4fc3_8bcf_e5140a52547a
| altLabel | RoundTripEfficiency |
| elucidation | ratio of the electric energy provided from a secondary battery [ or electrochemical device ] during discharge to the electric energy supplied to the battery during the preceding charge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-53 |
| hiddenLabel | EnergyEfficiency/% |
| subclassOf | RatioQuantity, ElectrochemicalPerformanceQuantity |
EqualizationCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_990d19b8_672a_4219_a2b3_0a25bfa13f69
| elucidation | extended charge to ensure an equal state of charge of all cells in a battery |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-40 |
| subclassOf | Charging |
EquilibriumElectrodePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d91940f0_c8b6_4505_9b68_6bf6cfc5c544
| altLabel | EquilibriumPotential; NernstPotential; ReversiblePotential |
| elucidation | potential of an electrode when no electric current flows through the cell and all local charge transfer equilibria across phase boundaries that are represented in the cell diagram (except at possible electrolyte-electrolyte junctions) and local chemical equilibria are established |
| wikipediaReference | https://en.wikipedia.org/wiki/Reversal_potential |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-12 |
| subclassOf | OpenCircuitVoltage, ElectrodePotential |
| subclasses | StandardElectrodePotential, FormalElectrodePotential |
ExchangeCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ccde24bb_790a_40ca_a06e_cea156a61031
| elucidation | the common value (i0) of the anodic and cathodic partial currents when the reaction is at equilibrium |
| iupacReference | https://goldbook.iupac.org/terms/view/E02238 |
| subclassOf | ElectricCurrent, ElectrochemicalKineticQuantity |
ExchangeCurrentDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e9fd9ef9_adfe_46cb_b2f9_4558468a25e7
| altLabel | MeanExchangeCurrentDensity |
| elucidation | defined by j0 = i0/A, where i0 is the exchange current of the electrode reaction and A is usually taken as the geometric area of the electrode |
| wikipediaReference | https://en.wikipedia.org/wiki/Exchange_current_density |
| iupacReference | https://goldbook.iupac.org/terms/view/M03777 |
| subclassOf | ElectricCurrentDensity, ElectrochemicalKineticQuantity |
ExchangeCurrentDensityData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b7ea60b2_18f4_4588_bf19_55539c8e8888
| elucidation | data that describes the exchange current density of some material or electrode under some stated conditions |
| subclassOf | Dataset |
ExpandedMesh#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_31b38a1b_5873_454c_ba99_00491497fb43
| elucidation | a sheet that has been slit and stretched to form a network of diamond-shaped openings, providing structural strength and high surface area for applications like filtration and reinforcement |
| subclassOf | ManufacturedMaterial |
FaradaicCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2a2f59b7_aa16_40aa_9c8b_0de8a2720456
| elucidation | electric current that results from the electrooxidation or electroreduction of an electroactive substance |
| wikidataReference | https://www.wikidata.org/wiki/Q5434687 |
| wikipediaReference | https://en.wikipedia.org/wiki/Faradaic_current |
| dbpediaReference | https://dbpedia.org/page/Faradaic_current |
| iupacReference | https://goldbook.iupac.org/terms/view/F02321 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
| subclasses | DiffusionCurrent, LimitingCurrent, CatalyticCurrent, KineticCurrent, NetFaradaicCurrent |
FaradayConstant#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_77f9d496_555e_4ae2_ae80_f297ef8335ca
| elucidation | fundamental physical constant representing molar elementary charge |
| wikidataReference | https://www.wikidata.org/wiki/Q192819 |
| wikipediaReference | https://en.wikipedia.org/wiki/Faraday_constant |
| iupacReference | https://doi.org/10.1351/goldbook.F02325 |
| subclassOf | SIExactConstant, ElectrochemicalQuantity |
FaradaysFirstLawOfElectrolysis#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1152ae6b_8b57_4d99_912e_40c6a29342fb
| altLabel | FaradaysLaw; FaradaysLawOfElectrolysis |
| elucidation | mass m of electrochemically-transformed substance is proportional to the charge Q passed, m ∝ Q. |
| wikipediaReference | https://en.wikipedia.org/wiki/Faraday%27s_laws_of_electrolysis#First_law |
| subclassOf | PhysicalLaw |
FaradaysSecondLawOfElectrolysis#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_60c5b2e5_164a_4ce6_8409_f386f5e50c03
| elucidation | when the same electric charge (quantity of electricity) Q is passed through several electrolytes, the mass, m_i, of the substances deposited are proportional to their respective chemical equivalent molar mass, M_i/z_i. |
| wikipediaReference | https://en.wikipedia.org/wiki/Faraday%27s_laws_of_electrolysis#Second_law |
| subclassOf | PhysicalLaw |
Note
m_1/m_2 = (M_1/z_1) / (M_2/z_2)
FinishingCRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_50674621_09ae_4f03_8ee9_3997b88c8b2a
| altLabel | FinishingChargeRate |
| elucidation | [an indicator of the] electric current at which a battery is charged towards the end of charge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-46 |
| subclassOf | CRate |
FlameArrestorVent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ae56cce3_7a7c_4e53_bb58_31e0d642d47e
| elucidation | vent of special design which provides protection against the propagation of a flame front from or into the interior of a secondary cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-11 |
| subclassOf | Component |
Note
this flame front may originate from a spark or an external naked flame igniting combustible electrolysis gas
FloatCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3e7fe02d_c888_4c49_8e05_ccd9785607a6
| altLabel | ConstantVoltageCharging; PotentiostaticCharging |
| elucidation | charge during which the voltage is maintained at a constant value regardless of charge current or temperature |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-49 |
| restrictions | |
| subclassOf | Charging, VoltageHold |
FlowCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_95e4aa95_b7e9_40d7_b78e_4d7dcc31093d
| altLabel | ElectrochemicalFlowCell |
| elucidation | an electrochemical device in which the active materials are flowed through the cell from an external source |
| restrictions |
|
| subclassOf | ElectrochemicalDevice |
| subclasses | Electrolyser, HybridFlowCell, FuelCell |
FlowField#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b87124e6_bc69_4216_ab48_2140249801a1
| elucidation | a flow field is the structured channel network that distributes reactants across the electrode surface and facilitates the removal of products. |
| subclassOf | Component |
Note
Flow field design affects pressure drop, mass transport, and overall cell performance.
FluorineDopedTinOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_339e1ba1_2006_4f22_aac4_3ce75edf1088
| altLabel | FTOElectrode |
| elucidation | electrode in which the active material is tin oxide doped with fluorine |
| restrictions | |
| subclassOf | MetalOxideElectrode |
Foil#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1d5377a7_9f2b_4fdf_958a_7eeadce158d6
| elucidation | a sheet of material in which the thickness is much smaller than the length and width |
| subclassOf | ManufacturedMaterial |
FormFactor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1586ef26_6d30_49e3_ae32_b4c9fc181941
| elucidation | a sign that is used to indicate the shape of the case of an electrochemical device |
| subclassOf | Information |
| subclasses | Prismatic, Coin, Cylindrical, Pouch |
FormalElectrodePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b21de1ef_6c15_4d63_b320_c9b96fbf186f
| altLabel | FormalPotential |
| elucidation | equilibrium electrode potential under conditions of unit concentration of species involved in the electrode reaction |
| subclassOf | EquilibriumElectrodePotential |
FormationCycling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cb223440_51bd_4f16_a536_96ec408e7de4
| elucidation | the cycling of a cell after manufacturing, usually for the purpose of forming interfacial layers |
| subclassOf | Cycling |
FrequencyResponseAnalyser#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_279ecc9f_bfbc_4108_ae40_3c1c0f735e60
| altLabel | FRA; FrequencyResponseAnalyzer |
| elucidation | analyzes the output signal of a stimulated system |
| subclassOf | Device |
| subclasses | MTZ35 |
Note
based on the Fourier Transform analysis
FuelCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bd33779c_6f40_4354_ab5d_f6c17396414d
| elucidation | galvanic cell that transforms chemical energy from continuously supplied reactants to electric energy by an electrochemical process. |
| wikidataReference | https://www.wikidata.org/wiki/Q180253 |
| wikipediaReference | https://en.wikipedia.org/wiki/Fuel_cell |
| dbpediaReference | https://dbpedia.org/page/Fuel_cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-05 |
| subclassOf | FlowCell |
GalvanicCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e248373f_294f_4ca4_9edf_0ad6653bb64f
| elucidation | electrochemical cell in which chemical reactions occur spontaneously and chemical energy is converted into electrical energy. |
| wikidataReference | https://www.wikidata.org/wiki/Q209440 |
| wikipediaReference | https://en.wikipedia.org/wiki/Galvanic_cell |
| dbpediaReference | https://dbpedia.org/page/Galvanic_cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-02 |
| subclassOf | ElectrochemicalCell |
Note
An electrochemical cell that spontaneously produces work.
Note
Electrochemical cell intended to produce electric energy.
Galvanostat#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_22725105_c941_4b14_a4a2_fcb627958607
| altLabel | Amperostat |
| elucidation | instrument which controls the electric current between the working electrode and the auxiliary electrode |
| wikidataReference | https://www.wikidata.org/wiki/Q5519411 |
| wikipediaReference | https://en.wikipedia.org/wiki/Galvanostat |
| subclassOf | Device |
| subclasses | VMP3e, VSP3e |
GalvanostaticCycling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_79a8802e_8296_459a_b65d_6e5c79e1bf37
| elucidation | a cycling protocol in which an electrochemical cell is subject to alternating galvanostatic charging and discharging processes |
| restrictions | |
| subclassOf | CurrentControlledProcess, Cycling |
GalvanostaticIntermittentTitrationTechniqueData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ac2ffdd9_09cf_42a7_ba6a_1c6968e9a8a5
| altLabel | GITTData |
| elucidation | a dataset resulting from a galvanostatic intermittent titration technique experiment or simulation |
| subclassOf | TimeSeriesElectricalDataSet |
GasCompartment#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_14df0300_de16_4298_94ae_48652e250b78
| elucidation | an internal compartment of the electrochemical flow cell through which gas flows |
| subclassOf | Component |
GasDiffusionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bbb1d95b_72d1_44f6_b07e_a3d7d41ac215
| altLabel | GDE |
| elucidation | a type of electrode specifically designed for gaseous reactants or products or both |
| wikidataReference | https://www.wikidata.org/wiki/Q909759 |
| wikipediaReference | https://en.wikipedia.org/wiki/Gas_diffusion_electrode |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=485-02-02 |
| restrictions | |
| subclassOf | PorousElectrode, InertElectrode |
| subclasses | OxygenElectrode, AirElectrode, CardonDioxideElectrode |
Note
A gas diffusion electrode usually comprises one or more porous layers, like the gas diffusion layer and the catalyst layer.
Note
Gas diffusion electrodes can be gas diffusion anodes or gas diffusion cathodes.
Note
Gas diffusion electrodes can be used in batteries, fuel cells, and electrolyzers.
GasDissolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0c9017b4_6efd_4e2b_8e24_48b60ebe9315
| elucidation | the dissolution of molecules from a gas into another phase |
| subclassOf | Dissolution |
Note
Often leads to efficiency loss or mechanical degradation in electrochemical devices.
Example
Absorption of CO₂ into an alkaline electrolyte forming carbonate species.
Example
Hydrogen evolution during water electrolysis.
GasEvolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4288b145_ba79_4989_92f8_86086679b0fe
| altLabel | Gassing |
| elucidation | process through which a gas is evolved |
| wikidataReference | https://www.wikidata.org/wiki/Q5526337 |
| wikipediaReference | https://en.wikipedia.org/wiki/Gas_evolution_reaction |
| subclassOf | ChemicalReaction |
GassingOfACell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0f827b54_370d_4c63_99a6_80f13b24e55e
| elucidation | evolution of gas resulting from electrolysis of the water in the electrolyte of a cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-51 |
| restrictions | |
| subclassOf | ElectrochemicalPhenomenon |
GlassFibreSeparator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d11b0e66_c35b_4da7_80a1_037ce89b77fb
| altLabel | FibreglassSeparator |
| elucidation | a separator is composed of a mass of intermeshed glass fibers. |
| subclassOf | Separator |
GlassyCarbonElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_057bb143_639c_472b_99ed_ffa1867f6e63
| altLabel | GCE |
| elucidation | electrode made of glassy carbon material with an intertwined graphitic ribbon structure, formed by pyrolysis of a resol precursor at temperatures up to 3000 °C |
| subclassOf | InertElectrode |
Note
extremely resistant to many chemicals, impermeable to gases and liquids, and has good electrical conductivity, high hardness and strength, and biocompatibility
Note
glassy carbon combines glass-like mechanical characteristics with physical properties similar to graphite, owing to its “graphene ribbon” structure
Note
one of the most frequently used working electrodes
GoldBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_52547692_f773_4e3f_8c8b_1d9d39bc3c8c
| elucidation | an electrode in which the primary active material consists of gold or gold compounds |
| subclassOf | ActiveElectrode |
| subclasses | GoldElectrode |
GoldElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6fec8cc1_4e6c_428e_8343_6cf3c286a185
| altLabel | AuElectrode |
| elucidation | foil, wire or disc electrode made of gold which is easily fabricated into a variety of electrode geometries |
| restrictions |
|
| subclassOf | GoldBasedElectrode |
Note
At positive potentials, Au forms an oxide and/or chemisorbed oxygen layer, while in the presence of complexing anions, such as chloride or cyanide, it readily undergoes oxidation and dissolution, limiting its working potential range and its applications.
GouyChapmanModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fa54f95d_b49e_43b5_84c3_35520d0fb2f6
| elucidation | an extension of the Helmholtz model that describes the distribution of ions as a function of distance from the electrode surface to the bulk of the electrolyte |
| subclassOf | DoubleLayerModel |
Note
this model fails for highly charged double layers
GrahameModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7f168ebc_4c78_459c_9a39_bddaa57e214c
| elucidation | a modification of the Stern model that considers the double layer as three regions separated by the inner Helmholtz plane and the outer Helmholtz plane |
| subclassOf | DoubleLayerModel |
Note
proposed by D. C. Grahame in 1947
GraphiteElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c831d963_629a_41ab_850f_97fb6841b739
| elucidation | electrode in which the active material is graphite |
| restrictions | |
| subclassOf | ActiveElectrode, CarbonBasedElectrode |
GuestDiffusivityInNegativeElectrodeActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_50247e71_75fe_4986_959e_fd06c6be98db
| elucidation | the diffusivity of lithium in the negative electrode |
| bpxKey | ['Parameterisation','Negative electrode','Diffusivity [m2.s-1]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'diffusion_constant','value'] |
| subclassOf | Diffusivity |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
GuestDiffusivityInPositiveElectrodeActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e59188bb_ce66_49f6_84aa_ecb98e76941e
| elucidation | the diffusivity of lithium in the positive electrode |
| bpxKey | ['Parameterisation','Positive electrode','Diffusivity [m2.s-1]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'diffusion_constant','value'] |
| subclassOf | Diffusivity |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
HCP1005#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8a881d91_83af_4583_9210_c766d94d944e
| altLabel | HCP-1005 |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
Note
specifically designed to study high-capacity secondary batteries
HCP803#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6f71d385_a79f_42aa_89ef_2edb1ba05b61
| altLabel | HCP-803 |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
HalfPeakPotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c4d551db_db17_4c33_92e6_aec71638afbc
| elucidation | for dynamic voltammetric techniques, the electric potential of the working electrode at which the current is equal to one-half of the peak current |
| iupacReference | https://doi.org/10.1351/goldbook.H02720 |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
HalfWavePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2ae53fc6_d44d_41c9_acaf_c5606e6a981d
| elucidation | electric potential of a steady-state voltammetric working electrode at the point, on the rising part of the voltammetric wave, where the current is equal to one-half of the limiting current |
| iupacReference | https://doi.org/10.1351/goldbook.H02722 |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
HardCarbonElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6235cc7c_2eee_432a_93af_47d7e05db007
| elucidation | electrode in which the active material is hard carbon |
| restrictions | |
| subclassOf | ActiveElectrode, CarbonBasedElectrode |
HelmholtzModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9bc02662_9799_4593_906d_638a841d7352
| elucidation | a structural representation of the double layer that states that the accumulation of electric charge on the surface of the electrode is balanced by a layer of ions in the electrolyte |
| subclassOf | DoubleLayerModel |
Note
proposed by Hermann von Helmholtz in 1853
Note
the first model for the double layer
HermeticallySealedCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c14ae9b3_e4ba_4129_a7b6_afd2d4571de6
| elucidation | permanently sealed gas tight cell without pressure release device |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-01 |
| subclassOf | ElectrochemicalDevice |
HybridFlowCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f54474fc_5d07_474b_97ae_f5d0349363b4
| elucidation | a flow cell that uses one or more electroactive components deposited as a solid layer |
| subclassOf | FlowCell |
HydrogenElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c4a778c7_33da_4e1a_960e_402a210bfeff
| elucidation | platinized platinum electrode saturated by a stream of pure gaseous hydrogen |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-16 |
| subclassOf | InertElectrode |
Note
A platinized platinum electrode consists of a platinum rod covered by compact platinum powder called platinum black.
ImaginaryElectricImpedance#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0089729b_5890_4c15_aa09_1244d41f8626
| elucidation | the imaginary part of the complex electric impedance |
| subclassOf | ElectricImpedance |
InactiveMassLoading#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2cfea66b_756d_4642_986f_9a41abbd7034
| elucidation | the mass of inactive material per unit area |
| subclassOf | MassLoading |
IncreasingPotentialPulses#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_77be01ef_49bd_4f2e_bec8_eec0894b8562
| elucidation | Pulses of electric potential, of amplitude increasing by a constant increment and with a pulse width of 2 to 200 ms, which are superimposed on a constant initial potential. |
| subclassOf | ElectricPotentialSignal |
IndicatorElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f6fcd255_248d_4603_b128_04dab960a676
| elucidation | electrode that responds to one, or more than one, species in the solution being investigated, with no appreciable change of bulk solution composition during the measurement |
| wikidataReference | https://www.wikidata.org/wiki/Q120907462 |
| iupacReference | https://goldbook.iupac.org/terms/view/I03006 |
| subclassOf | Electrode |
Note
If processes of interest occur both at the anode and the cathode of a cell (as in differential amperometry or controlled-current potentiometric titration with two indicator electrodes), the cell should be said to comprise two indicator or working electrodes.
Note
This term is typically used in potentiometry.
IndiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7b9db6b3_36f0_4b5d_acbb_9284a9054a09
| elucidation | an electrode in which the primary active material consists of indium or indium compounds |
| subclassOf | ActiveElectrode |
IndiumTinOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c63baed9_48dd_4b5c_9e8c_03011010ffb6
| altLabel | ITO |
| elucidation | electrode in which the active material is indium tin oxide |
| restrictions | |
| subclassOf | BimetallicOxideElectrode |
InertElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a0a619d7_da95_41f0_8bc3_9c19d636d543
| elucidation | electrode that serves only as a source or sink for electrons without playing a chemical role in the electrode reaction |
| wikidataReference | https://www.wikidata.org/wiki/Q120907475 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-08 |
| subclassOf | Electrode |
| subclasses | GasDiffusionElectrode, GlassyCarbonElectrode, CarbonPasteElectrode, HydrogenElectrode |
Note
Noble metals, mercury, and carbon are typically used as (nearly) inert electrodes.
InitialChargeCarrierConcentrationInElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_098f98dc_e015_4dbd_b358_a7ac3b3ecff3
| elucidation | the initial amount concentration of lithium in an electrolyte |
| bpxKey | ['Parameterisation','Electrolyte','Initial concentration [mol.m-3]'] |
| cidemodKey | ['electrolyte','initial_concentration','value'] |
| subclassOf | AmountConcentration |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
InitialCondition#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2c4c2626_7db5_440c_a65e_95ed2986ee01
| elucidation | the initial value of a property at the start of a simulation or experiment |
| subclassOf | ControlProperty |
InitialDischargingVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_74861991_7da2_4e0f_b6c1_b16713f538bf
| altLabel | InitialDischargeVoltage; InitialClosedCircuitVoltage |
| elucidation | discharge voltage of a cell or battery at the beginning of the discharge immediately after any transients have subsided |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-29 |
| subclassOf | DischargingVoltage |
InititalThermodynamicTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9c9b80a4_a00b_4b91_8e17_3a7831f2bf2f
| elucidation | the thermodynamic temperature at some point in time designated to be the initial state |
| subclassOf | ThermodynamicTemperature |
InsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_757eae08_4d43_42d4_8b4e_8a0bfd2f9a1c
| altLabel | IntercalationElectrode |
| elucidation | an electrode at which the predominant electrochemical reaction includes an insertion (intercalation) step |
| restrictions | |
| subclassOf | ActiveElectrode |
| subclasses | ZincInsertionElectrode, CalciumInsertionElectrode, PotassiumInsertionElectrode, SodiumInsertionElectrode, MagnesiumInsertionElectrode, ProtonInsertionElectrode, AluminiumInsertionElectrode, LithiumInsertionElectrode |
InstantaneousCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a24f8581_a5a4_41a4_bb45_d0fdd5c0d810
| elucidation | value of an electric current at an instant in time |
| wikidataReference | https://www.wikidata.org/wiki/Q108057717 |
| iupacReference | https://goldbook.iupac.org/terms/view/I03062 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
| subclasses | CellCurrent |
InstantaneousPower#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5973f91a_fa6b_49c9_ba46_3ecc1c2253d2
| elucidation | for a two-terminal element or a two-terminal circuit with terminals A and B, product of the voltage uAB between the terminals and the electric current i in the element or circuit |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=131-11-30 |
| newareLabel | Power(mW) |
| indigoLabel | cell_power_w |
| subclassOf | Power |
Interface#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_94d9d5e2_9e9b_4eb7_b3ce_f3d658b7ae64
| elucidation | the plane ideally marking the boundary between two phases |
| wikidataReference | https://www.wikidata.org/wiki/Q901882 |
| wikipediaReference | https://en.wikipedia.org/wiki/Interface_(matter) |
| iupacReference | https://doi.org/10.1351/goldbook.I03082 |
| illustration |  |
| subclassOf | NonTemporalRole |
| subclasses | ElectrochemicalInterface |
InterfacialRegion#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f78e3c87_01c6_4cc2_b047_30322262e22d
| elucidation | a 3D spatial region at an interface with physical properties that are distinct from the bulk phases in contact |
| illustration | 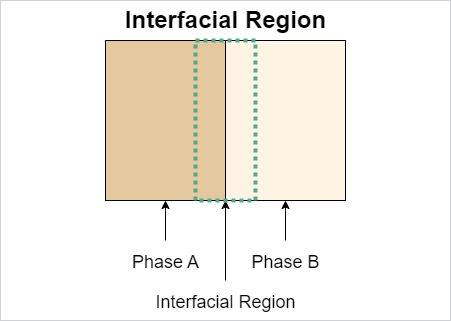 |
| subclassOf | NonTemporalRole |
| subclasses | DoubleLayer, SpaceChargeLayer, Interphase, PassivationLayer |
InternalApparentResistance#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f94678d6_1386_48fc_8e54_024921924401
| elucidation | quotient of change of voltage of a battery by the corresponding change in discharge current under specified conditions. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-36 |
| subclassOf | InternalResistance |
InternalConductance#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0c9655c6_6b0b_4819_a219_f286ad196fa9
| elucidation | the internal conductance of an electrochemical device |
| subclassOf | ElectricConductance, ElectrochemicalTransportQuantity |
InternalResistance#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9bf40017_3f58_4030_ada7_cb37a3dfda2d
| elucidation | impedance of a linear circuit's Thévenin representation |
| wikidataReference | https://www.wikidata.org/wiki/Q2527701 |
| wikipediaReference | https://en.wikipedia.org/wiki/Internal_resistance |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=485-15-04 |
| maccorLabel | dcir/ohms |
| newareLabel | DCIR(O) |
| arbinLabel | internal_resistance |
| subclassOf | ElectricResistance, ElectrochemicalPerformanceQuantity |
| subclasses | InternalApparentResistance |
Interphase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_54e0c2bd_1bb2_4f9c_9b55_5b6cc34651ec
| altLabel | InterfacialLayer; InterfacialRegion |
| elucidation | a spatial region at the interface between two bulk phases in contact, which is different chemically and physically from both phases in contact |
| iupacReference | https://goldbook.iupac.org/terms/view/I03085 |
| illustration | 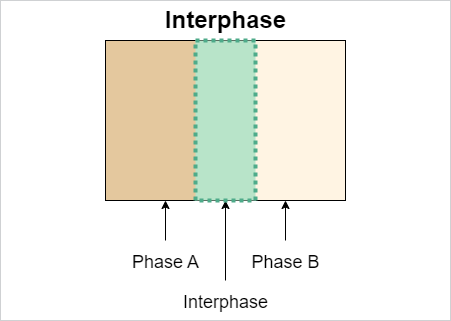 |
| subclassOf | InterfacialRegion |
Example
SEI
InterphaseGrowth#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_402598d9_0eea_405d_b0d0_3b8b79deba6f
| elucidation | the process through which the size of an interphase increases over time |
| restrictions |
|
| subclassOf | ElectrochemicalPhenomenon |
Example
solid electrolyte interphase (SEI) growth
IonConcentration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8177eae6_1631_430d_99f2_942669bcb784
| elucidation | the amount concentration of some ionic species in a given volume of solution |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-01-16 |
| subclassOf | AmountConcentration, ElectrochemicalThermodynamicQuantity |
IonExchangeMembrane#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_15325775_6d4c_4192_82f1_e4feca9de426
| elucidation | A membrane having ion-exchange groups: |
| wikidataReference | https://www.wikidata.org/wiki/Q21082486 |
| wikipediaReference | https://en.wikipedia.org/wiki/Ion-exchange_membrane |
| iupacReference | https://doi.org/10.1351/goldbook.IT07155 |
| subclassOf | Separator |
| subclasses | AnionExchangeMembrane, CationExchangeMembrane |
Note
a selective barrier that permits the passage of ions of one sign while preventing the passage of ions of opposite sign.
IonicConductivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_25dabdc2_68bf_4a38_8cbe_11be017358bc
| elucidation | a measure of a substance's tendency towards ionic conduction |
| wikidataReference | https://www.wikidata.org/wiki/Q6063658 |
| wikipediaReference | https://en.wikipedia.org/wiki/Ionic_conductivity_(solid_state) |
| iupacReference | https://doi.org/10.1351/goldbook.I03175 |
| subclassOf | ElectricConductivity, ElectrochemicalTransportQuantity |
| subclasses | ElectrolyticConductivity |
IonicLiquidElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c3f4b34a_0e2c_46f3_baab_4ebd2682d26f
| altLabel | IonicLiquid |
| elucidation | An ionic liquid is an electrolyte composed of a salt that is liquid below 100 °C. Ionic liquids have found uses in electrochemical analysis, because their unconventional properties include a negligible vapor pressure, a high thermal and electrochemical stability, and exceptional dissolution properties for both organic and inorganic chemical species. |
| wikipediaReference | https://en.wikipedia.org/wiki/Ionic_liquid |
| dbpediaReference | https://dbpedia.org/page/Ionic_liquid |
| subclassOf | NonAqueousElectrolyte |
IridiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3e6a7d5f_3700_46b3_b1b8_f34e37e6f931
| elucidation | an electrode in which the primary active material consists of iridium or iridium compounds |
| subclassOf | ActiveElectrode |
IridiumOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cba03815_eaa8_493d_b9e4_52856b57fde6
| altLabel | IrO2Electrode |
| elucidation | electrode in which the active material is iridium oxide |
| restrictions | |
| subclassOf | MetalOxideElectrode |
IronBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cc4e178c_bc1f_4502_b6c2_33f304ef6bab
| elucidation | an electrode in which the primary active material consists of iron or iron compounds |
| subclassOf | ActiveElectrode |
IronBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5e1136d3_df00_40f7_a4bc_8259341053a1
| elucidation | an electrode which contains mostly materials based on iron |
| subclassOf | ActiveElectrode |
| subclasses | IronDisulfideElectrode |
Note
often represented in cell designations by the letter F
Note
this class is intended to enable designations based on IEC recommendations
IronDisulfideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d2726dd5_69f0_4cb1_bd3c_4c48813e57e7
| elucidation | an electrode in which the primary active material consists of iron disulfide or iron disulfide compounds. |
| restrictions | |
| subclassOf | IronBasedElectrode, ActiveElectrode |
Note
the combination with lithium metal and an organic electrolyte is designated using IEC electrochemical system letter code F
IronPhosphateBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d54f6aac_7cd2_4d52_9bca_2f19bb1eaec4
| elucidation | an electrode in which the primary active material consists of iron phosphate or iron phosphate compounds |
| subclassOf | ActiveElectrode |
| subclasses | LithiumIronPhosphateElectrode, SodiumIronPhosphateElectrode |
Note
represented in IEC cell designations by the letter code Fp
IsopotentialPoint#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ff7797ed_9ef7_40d0_872b_2c215cd54578
| elucidation | electric potential of an ion-selective electrode (ISE) and activity of an analyte ion at which the potential of the ISE is independent of temperature |
| iupacReference | https://doi.org/10.1351/goldbook.I03304 |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
Jacket#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a0fcb105_587f_45d8_a89a_46dc8f745069
| elucidation | partial or complete external covering of a cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-20 |
| subclassOf | Component |
Note
a jacket may be made of metal (insulated from the cell terminals), plastic, paper or other suitable material
KineticCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_98b6e9d7_d5df_46a5_87dd_79642b8b2e4b
| elucidation | faradaic current of an electroactive substance B formed by a prior chemical reaction from another substance Y that is no electroactive at the potential at which B is electrochemically transformed |
| iupacReference | https://goldbook.iupac.org/terms/view/K03399 |
| subclassOf | FaradaicCurrent |
KohlrauschsLaw#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_598ae3d0_76e9_429c_a0e1_8694525cb574
| elucidation | For any electrolyte A_xB_y, the limiting molar conductivity is expressed as x times the limiting molar conductivity of A^{y+} and y times the limiting molar conductivity of B^{x-}. |
| wikidataReference | https://www.wikidata.org/wiki/Q3258329 |
| wikipediaReference | https://en.wikipedia.org/wiki/Molar_conductivity#Kohlrausch's_law_of_independent_migration_of_ions |
| subclassOf | MaterialLaw |
Note
proposed by Friedrich Kohlrausch between 1875-1879
Note
valid for dilute solutions
LF100#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_933234b6_43f0_46ed_bc1c_3751f8bc7fe5
| elucidation | An electroltte with the nominal recipeEC/PC/DMC (1/1/3,wt/wt/wt) + LiFSI (13.74%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP100#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_826f50dc_e01e_4bb1_b6f5_183b58478cde
| elucidation | An electrolyte with the nominal capacity EC/PC/DMC (1/1/3,wt/wt/wt) + LiPF6 (11.46%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP30#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_29c4a743_e39e_4c32_b933_2213fe8d7625
| elucidation | An electrolyte mixutre with the nominal recipe EC/DMC (1/1, wt/wt) + LiPF6 (11.8%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP40#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_750eba5a_2a26_4a2b_8a2b_c09ae185a432
| elucidation | An electrolyte with the nominal recipe EC/DEC (1/1, wt/wt) + LiPF6 (12.4%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP47#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_464ef51d_0484_4477_8fab_869b658023fe
| elucidation | An electrolyte with the nominal recipe EC/DEC (3/7, wt/wt) + LiPF6 (13.1%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP50#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bad4fdeb_5290_4fa1_8b49_193f7762dce4
| elucidation | An electrolyte with the nominal recipe EC/EMC (1/1, wt/wt) + LiPF6 (12.2%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP57#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6b0d3c26_821a_4881_9ebd_fb439c190183
| elucidation | An electrolyte with the nominal recipe EC/EMC (3/7, wt/wt) + LiPF6 (12.7%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LP71#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_596a1b64_f5ac_4fe1_b5ae_a6e9845d3bab
| elucidation | An electrolyte with the nominal recipe EC/DEC/DMC (1/1/1, wt/wt/wt) + LiPF6 (12.4%,wt) |
| etymology | the label originated as a commercial/product code used by electrolyte suppliers and subsequently adopted as informal community shorthand in publications and laboratory practice |
| subclassOf | OrganicElectrolyte |
Caution
not an IEC, IUPAC, or ISO standardized designation; do not infer composition from the code alone; the same label is sometimes used with small vendor- or era-dependent variations
LawOfMassAction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5a7a3028_db9e_4045_ab68_054c6afc91fc
| elucidation | the rate of the chemical reaction is directly proportional to the product of the activities or concentrations of the reactants |
| wikidataReference | https://www.wikidata.org/wiki/Q899494 |
| wikipediaReference | https://en.wikipedia.org/wiki/Law_of_mass_action |
| subclassOf | PhysicalLaw |
LeadBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_610f0bc8_557d_455b_a8ed_272d5d1813c9
| elucidation | an electrode in which the primary active material consists of lead or lead compounds |
| subclassOf | ActiveElectrode |
LeadOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f6223121_7df5_42f8_902c_d26fa2fc4f04
| altLabel | PbOElectrode |
| elucidation | electrode in which the active material is lead oxide |
| restrictions | |
| subclassOf | MetalOxideElectrode |
Leakage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c55ec02c_b727_4df1_97ce_91d7bb5d5e91
| elucidation | unplanned escape of electrolyte, gas or other material from a cell or battery |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-32 |
| subclassOf | ElectrochemicalPhenomenon |
LeclancheElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_23b30468_be7b_45a9_bc11_a8219bc3ab8b
| elucidation | an aqueous mixture of ammonium chloride and zinc chloride |
| subclassOf | NearNeutralElectrolyte |
LidSealingCompound#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6559c04d_75bc_41ea_8ed8_8d3c09dae6b0
| elucidation | material used to seal the cell lid to the case or to the terminals of a cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-16 |
| subclassOf | CompositePhysicalObject |
LimitQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b395a244_3a75_4737_be38_981bfa1277fe
| elucidation | a limit quantity in a control system |
| subclassOf | ElectrochemicalControlQuantity |
| subclasses | LowerCRateLimit, TemperatureLimit, VoltageLimit, ConcentrationLimit, CurrentLimit |
LimitingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d5ac8868_d318_4065_aa23_72140ae888ae
| elucidation | faradaic current that is approached as the rate of the charge-transfer process is increased by varying the applied potential, being greater than the rate of mass transport controlled by diffusion |
| iupacReference | https://goldbook.iupac.org/terms/view/L03532 |
| subclassOf | FaradaicCurrent |
LimitingCurrentDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_077de429_ea41_444c_8571_6a832ae62637
| elucidation | maximum electric current density that can be achieved for an electrode reaction at a given concentration of a electrochemically active material in the presence of a large excess of supporting electrolyte |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-13 |
| subclassOf | ElectricCurrentDensity |
LimitingMolarConductivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a17ee4e0_c81a_4a64_9ecb_9c6fa022cf4d
| elucidation | molar conductivity at infinite dilution |
| wikipediaReference | https://en.wikipedia.org/wiki/Molar_conductivity#Variation_of_molar_conductivity_with_dilution |
| subclassOf | MolarConductivity, ElectrochemicalTransportQuantity |
LinearCurrentRamp#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fa774456_718a_4a28_ba6a_6f828887c510
| elucidation | signal where an electric current is varied linearly from an initial current, typically 0 A |
| subclassOf | ElectricCurrentSignal |
LinearPotentialRamp#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_29f2a35a_8c09_429d_b9e8_33f3e1fc3671
| elucidation | Signal where the electric potential is varied linearly from an initial potential. |
| subclassOf | ElectricPotentialSignal |
LiquidElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_609b340f_3450_4a10_95c2_c457e3eb8a89
| elucidation | an electrolyte in the liquid phase. |
| restrictions | |
| subclassOf | Electrolyte |
| subclasses | NonAqueousElectrolyte, ElectrolyteSolution |
LiquidGalliumElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_80fdbd63_9b7a_462b_a8cb_b50f5f6ab182
| elucidation | a liquid electrode with an active material of gallium |
| restrictions |
|
| subclassOf | LiquidMetalElectrode |
LiquidJunction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_634467ad_feed_4979_adb2_877d98fe1768
| elucidation | any junction between two electrolyte solutions of different composition. |
| iupacReference | https://goldbook.iupac.org/terms/view/L03584 |
| subclassOf | ElectrochemicalInterface |
Note
Across such a junction there arises a potential difference, called the liquid junction potential.
Note
In the operational pH cell the junction is between the test, or pH standard, solution and the filling solution or the bridge solution of the reference electrode.
LiquidMetalElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9fd49892_cf6d_482e_a6c3_5f763948ec29
| elucidation | an electrode that uses a liquid metal |
| wikidataReference | https://www.wikidata.org/wiki/Q6557456 |
| wikipediaReference | https://en.wikipedia.org/wiki/Liquid_metal_electrode |
| subclassOf | MetalElectrode |
| subclasses | LiquidGalliumElectrode, MercuryElectrode |
Note
they can be used in electrocapillarity, voltammetry, and impedance measurements
LithiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_47346d85_b9be_4480_8993_6307b1c58fcd
| elucidation | an electrode in which the primary active material consists of lithium or lithium compounds |
| subclassOf | ActiveElectrode |
| subclasses | LithiumElectrode |
LithiumCobaltOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fd7caf39_0a43_4fbf_958e_a62067aa9007
| altLabel | LCOElectrode |
| elucidation | electrode in which the active material is lithium cobalt oxide |
| restrictions | |
| subclassOf | CobaltBasedElectrode, MetalOxideElectrode |
LithiumElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_55775b50_b9d9_4d68_8cb5_38fcd7b9b54d
| elucidation | an electrode in which the active material is lithium metal |
| restrictions |
|
| subclassOf | LithiumBasedElectrode |
LithiumHydroxideSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e84e691a_df58_465c_9771_7a7fe2212ed5
| altLabel | AqueousLithiumHydroxideSolution; LiOHSolution |
| elucidation | a solution of lithium hydroxide (LiOH) dissolved in water (H2O) |
| subclassOf | AlkalineElectrolyte |
LithiumInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4c3ee364_829b_41a4_b895_ca4a041efb2a
| elucidation | an insertion electrode in which the guest molecule is lithium |
| subclassOf | InsertionElectrode |
LithiumIronPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1d0f15cb_d6b5_4c27_89ca_fe78adc1ce5b
| altLabel | LFPElectrode |
| elucidation | electrode in which the active material is lithium iron phosphate |
| restrictions | |
| subclassOf | IronPhosphateBasedElectrode, MetalOxideElectrode |
LithiumManganeseIronPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9109b3f6_112b_456d_ae45_b82c271c656b
| altLabel | LMFPElectrode |
| elucidation | electrode in which the active material is lithium manganese iron phosphate |
| subclassOf | ManganesePhosphateBasedElectrode, MetalOxideElectrode |
LithiumManganeseOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c231227b_f318_4e59_ad90_6e91072903ed
| altLabel | LMOElectrode |
| elucidation | electrode in which the active material is lithium manganese oxide |
| restrictions | |
| subclassOf | ManganeseBasedElectrode, MetalOxideElectrode |
LithiumManganeseOxideLithiumIronPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fffc3dad_6946_4c32_a9d3_f5f311339881
| altLabel | LMOLFPElectrode |
| elucidation | an electrode with blended lithium manganese oxide (LMO) and lithium iron phosphate (LFP) active materials |
| restrictions | |
| subclassOf | BimetallicOxideElectrode |
LithiumManganesePhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_713200a1_952b_49e2_90b7_deba229f6bbe
| altLabel | LMPElectrode |
| elucidation | electrode in which the active material is lithium manganese phosphate |
| subclassOf | ManganesePhosphateBasedElectrode, MetalOxideElectrode |
LithiumNickelCobaltAluminumOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f19d3b4f_d59b_4a92_a547_54a6f59cc9b4
| altLabel | NCAElectrode |
| elucidation | electrode in which the active material is lithium nickel cobalt aluminium oxide |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
LithiumNickelMananeseCobaltOxideLithiumManganeseOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_48e380c3_0441_4761_a80f_3e448cb2f0ba
| altLabel | NMCLMOElectrode |
| elucidation | an electrode with blended lithium nickel manganese cobalt oxide (NMC) and lithium manganese oxide (LMO) active materials |
| restrictions | |
| subclassOf | BimetallicOxideElectrode |
LithiumNickelManganeseCobaltOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b3eb8c65_5644_45e3_9e17_0be6277c7962
| altLabel | NMCElectrode |
| elucidation | electrode in which the active material is lithium nickel manganese cobalt |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
LithiumNickelManganeseCobaltOxideLithiumCobaltOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_098690e3_9010_437e_8042_cee1656efa9b
| altLabel | NMCLCOElectrode |
| elucidation | an electrode with blended lithium nickel manganese cobalt oxide (NMC) and lithium cobalt oxide (LCO) active materials |
| restrictions | |
| subclassOf | BimetallicOxideElectrode |
LithiumNickelManganeseOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_41ddf723_245f_4ce8_b9b1_7d9b3b9eea7d
| altLabel | LNMOElectrode |
| elucidation | electrode in which the active material is lithium nickel manganese oxide |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
LithiumNickelManganeseOxideLithiumIronPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bb710d71_98fa_48a1_85bc_d8f1ef2200da
| altLabel | LNMOLFPElectrode |
| elucidation | an electrode with blended lithium nickel manganese oxide (LNMO) and lithium iron phosphate (LFP) active materials |
| restrictions | |
| subclassOf | BimetallicOxideElectrode |
LithiumNickelOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cab66388_3feb_4101_82bc_f4441f0b60e3
| altLabel | LNOElectrode |
| elucidation | electrode in which the active material is lithium nickel oxide |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
LithiumTitanateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6d0fe07e_a629_479c_ab24_2846f209bb0b
| altLabel | LTOElectrode |
| elucidation | electrode in which the active material is lithium titanate |
| restrictions | |
| subclassOf | TitaniumBasedElectrode, MetalOxideElectrode |
LithiumVanadiumOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b5e91259_cd97_4ed6_9ab2_4b18ef68a35a
| elucidation | electrode in which the active material is lithium vanadium oxide |
| subclassOf | VanadiumBasedElectrode, MetalOxideElectrode |
LowerCRateLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_08b6d07a_0f6c_4932_afbd_fb6b699164ae
| elucidation | the lower value for C-Rate that is used to indicate a change in an electrochemical measurement process |
| subclassOf | LimitQuantity, CRate |
LowerCurrentDensityLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_abdd3bbb_403a_4cb3_b522_50d027492a68
| elucidation | the lower bounding limit placed on the current density used in the definition of an electrochemical testing protocol |
| subclassOf | CurrentDensityLimit |
LowerCurrentLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7db845e7_0e5b_47d4_b954_2a2b8e85deea
| elucidation | the lower bounding limit on the current used in the definition of an electrochemical testing protocol |
| subclassOf | CurrentLimit |
LowerFrequencyLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b66d6553_6136_4754_902a_707e414210c2
| elucidation | the lower end of the interval of frequencies tested in impedimetry and related techniques |
| subclassOf | Frequency, ElectrochemicalControlQuantity |
LowerVoltageLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_534dd59c_904c_45d9_8550_ae9d2eb6bbc9
| altLabel | CutOffVoltage; DischargeCutoffVoltage; EndOfDischargeVoltage; EndPointVoltage; EndVoltage; FinalVoltage; LowerCutoffVoltage |
| elucidation | minimum voltage limit at which an applied excitation is altered or terminated |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-30 |
| subclassOf | VoltageLimit |
MTZ35#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0cb3d0cc_0b4f_41b4_8a80_47015c3ff5b8
| altLabel | MTZ-35 |
| elucidation | a frequency response analyser (FRA) manufactured by BioLogic |
| subclassOf | FrequencyResponseAnalyser |
MagnesiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2a177462_ff01_4b83_ab9f_032e93c9ec69
| elucidation | an electrode in which the primary active material consists of magnesium or magnesium compounds |
| subclassOf | ActiveElectrode |
MagnesiumInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d9888f1f_2226_4ce3_9cb3_91fd9bd1bf22
| elucidation | an insertion electrode in which the guest molecule is magnesium |
| subclassOf | InsertionElectrode |
ManganeseBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b1ac8d0c_a215_4e60_82b0_38272eff5131
| elucidation | an electrode in which the primary active material consists of manganese or manganese compounds |
| subclassOf | ActiveElectrode |
| subclasses | ManganeseDioxideElectrode, LithiumManganeseOxideElectrode, SodiumManganeseHexacyanoferrateElectrode, SodiumManganeseOxideElectrode |
Note
often represented in cell designations by the letter M
Note
this class is intended to enable designations based on IEC recommendations
ManganeseDioxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ca4d9efc_70be_441e_b358_d927aa4c36c4
| altLabel | MnO2Electrode |
| elucidation | an electrode in which the primary active material consists of manganese dioxide or manganese dioxide compounds. |
| restrictions | |
| subclassOf | ManganeseBasedElectrode, MetalOxideElectrode |
Note
the combination with lithium metal and an organic electrolyte is designated using IEC electrochemical system letter code C
Note
the combination with zinc metal and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code L
ManganesePhosphateBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_468b3b63_b62a_4110_8c7e_40fffd5fdfd6
| elucidation | an electrode in which the primary active material consists of manganese phosphate or manganese phosphate compounds |
| subclassOf | ActiveElectrode |
| subclasses | LithiumManganeseIronPhosphateElectrode, LithiumManganesePhosphateElectrode, SodiumManganesePhosphateElectrode |
Note
often represented in cell designations by the letter Mp
Note
this class is intended to enable designations based on IEC recommendations
MassLoading#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c955c089_6ee1_41a2_95fc_d534c5cfd3d5
| elucidation | mass of a material per unit area |
| subclassOf | AreaDensity, ElectrochemicalQuantity |
| subclasses | TotalMassLoading, InactiveMassLoading, ActiveMassLoading |
MaximumCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b7781ebc_90a7_4f19_997f_aed28dee1b01
| altLabel | MaximumStorageCapacity |
| elucidation | the maximum amount of electric charge that can be reversible stored within an electrochemical device |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | RatedCapacity, ElectrochemicalQuantity |
Note
Manufacturers define the value for 'maximum capacity' as the upper performance limit that a device in a batch will typically achieve. The maximum capacity is larger than the nominal capacity, and acts as an indicator of the spread in performacne in a given batch of devices.
Caution
For real applications, the maximum capacity is affected by environmental conditions, rate of operation, and state-of-heath and can deviate from the reported value.
MaximumChargingTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4a354510_4dc2_4803_8845_f4024a1a7260
| elucidation | the maximum allowable temperature of an electrochemical device during a charging process |
| subclassOf | MaximumTemperature |
MaximumConcentration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_47287d09_6108_45ca_ac65_8b9451b1065e
| elucidation | the maximum amount concentration of a species in a phase, either imposed or naturally occurring |
| subclassOf | ConcentrationLimit |
| subclasses | PositiveElectrodeActiveMaterialMaximumGuestConcentration, NegativeElectrodeActiveMaterialMaximumGuestConcentration |
MaximumContinuousChargingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_39d8a6ee_cd55_4855_8b5b_d42bef95ac78
| altLabel | MaximumContinuousChargeCurrent |
| elucidation | the maximum current approved for continuous charge for a given electrochemical device |
| subclassOf | CurrentLimit |
MaximumContinuousDischargingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ba7ac581_0e13_4815_b888_013c378932f5
| altLabel | MaximumContinuousDischargeCurrent |
| elucidation | the maximum current approved for continuous discharge for a given electrochemical device |
| subclassOf | CurrentLimit |
MaximumDischargingPulseDuration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d5dc0c1d_0926_4268_89f0_4519a326eabc
| elucidation | the maximum duration of a pulse discharge for a given electrochemical device |
| subclassOf | PulseDuration |
MaximumDischargingTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_de612af2_a029_4a02_8090_4a75ab13271d
| elucidation | the maximum allowable temperature of an electrochemical device during a discharging process |
| subclassOf | MaximumTemperature |
MaximumPulseChargingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1b2a7137_64d4_483a_8437_dcb3bedcb6da
| altLabel | MaximumPulseChargeCurrent |
| elucidation | the maximum current approved for pulse charge of an electrochemical device |
| subclassOf | CurrentLimit |
MaximumPulseDischargingCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3e54f9e3_a31d_4821_9bfb_ef953a42c35b
| altLabel | MaximumPulseDischargeCurrent |
| elucidation | the maximum current approved for pulse discharge of an electrochemical device |
| subclassOf | PulseCurrent, CurrentLimit |
MaximumStoichiometricCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_95ef8c3c_62fe_467f_b5d4_dd0cd18a7ea8
| elucidation | The maximum allowed value for stoichiometric coefficient for an entity in a chemical substance |
| subclassOf | StoichiometricCoefficient |
MaximumStorageEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1cdeedbf_7f9a_4e1f_a297_204a6e16b718
| elucidation | the maximum amount of energy that can be reversible stored within an electrochemical device, component, or material - operated at an infinitely low rate - based on its chemical composition and design |
| subclassOf | RatedEnergy |
MaximumStorageTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ea4d188_9701_4699_a5ca_812a98a9afa7
| elucidation | the maximum temperature at which an electrochemical device shall be stored |
| subclassOf | MaximumTemperature |
MaximumTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cc519a19_b8d5_4e3f_b893_3a884888ad79
| elucidation | the maximum allowable temperature |
| newareLabel | Max-T(C) |
| subclassOf | TemperatureLimit |
| subclasses | MaximumDischargingTemperature, MaximumStorageTemperature, MaximumChargingTemperature |
MeasurementDataPostProcessing#
IRI: https://w3id.org/emmo/domain/electrochemistry#MeasurementDataPostProcessing
| elucidation | Application of a post-processing model to signals through a software, in order to calculate the final characterisation property. |
| subclassOf | DataPostProcessing |
| subclasses | EnergyCalculation, CapacityCalculation |
Example
Analysis of SEM (or optical) images to gain additional information (image filtering/integration/averaging, microstructural analysis, grain size evaluation, Digital Image Correlation procedures, etc.)
Example
In nanoindentation testing, this is the Oliver-Pharr method, which allows calculating the elastic modulus and hardness of the sample by using the load and depth measured signals.
MembranePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_601ff226_59b9_460b_90f5_2593450d96fa
| altLabel | DonnanPotential; MembraneElectromotiveForce |
| elucidation | electric potential difference between two solutions separated by an ion-selective membrane in the absence of any electric current flowing through the membrane |
| iupacReference | https://doi.org/10.1351/goldbook.M03825 |
| subclassOf | ElectricPotential, ElectrochemicalThermodynamicQuantity |
MercuryElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_df78745e_f9db_4830_88f0_8ce074fcb8ff
| altLabel | HgElectrode |
| elucidation | liquid metal electrode used in polarography |
| restrictions |
|
| subclassOf | LiquidMetalElectrode |
| subclasses | DroppingMercuryElectrode |
Note
A mercury film electrode (MFE) or thin mercury film electrode (TMFE) is formed by coating a conducting substrate, usually glassy carbon, with a layer of mercury (thin film, amalgam, or array of microdrops), often by electrodeposition from a solution containing Hg(II). The electrode area is normally in the range of 0.1 to 0.5 cm2 and mercury film thickness typically ranges from 10 to 1000 nm, producing a film of large surface area-to-volume ratio that results in a high analyte pre-concentration during the deposition step of anodic stripping voltammetry. Compared to an HMDE, the MFE provides high sensitivity and resolution; however, it is somewhat less reproducible and more prone to interferences from intermetallic formation and surface-active substances.
Note
Liquid mercury is an ideal electrode material for negative potentials because of its high overpotential for hydrogen evolution (electrochemical reduction of hydroxonium cations from solution). However, mercury is readily oxidized, particularly in the presence of anions that form complexes or that pre- cipitate with Hg(I) or Hg(II) ions, and thus it is not suitable for use at positive potentials. The use of liquid mercury has largely been discontinued because of concerns about the toxicity of the element and its compounds.
MetalElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5adb91e0_ffe1_41f3_b779_c6966f65fb0e
| elucidation | an electrode in which the actve electrochemical material is a metal |
| subclassOf | ActiveElectrode |
| subclasses | ZincElectrode, SolidAmalgamElectrode, LiquidMetalElectrode, BimetallicElectrode |
Note
the term metal is meant to loosely cover alkali metals, alkaline earth metals, transition metals, post-transition metals, and metalloids
MetalOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e4b6cb36_4dac_49e3_871d_40bcfca943a5
MigrationCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_22cec04f_c7f3_4ff8_a34b_e512379c9dcb
| elucidation | component of electric current due to transport of ions in the electric field between the electrodes |
| iupacReference | https://goldbook.iupac.org/terms/view/M03921 |
| subclassOf | ElectricCurrent, ElectrochemicalTransportQuantity |
MinimumCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d3c0078e_c1d3_461e_873d_e5c3adf441c5
| altLabel | MiniumumAcceptedCapacity |
| elucidation | the minimum acceptable threshold value for the rated capacity of an electrochemical device, as stated by the manufacturer. |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | RatedCapacity |
Note
Manufacturers define the value for 'minimum capacity' as the lower performance limit, ensuring that every device meets a minimum standard. In practice, minimum capacity is often about 95-97% of the nominal capacity, acting as a guaranteed lower bound rather than an exact measurement.
Caution
For real applications, the achieveable minimum capacity is affected by environmental conditions, rate of operation, and state-of-heath and can deviate from the reported value.
MinimumChargingTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b90b1ad7_b9a8_44df_ad45_bfd25aac2e49
| elucidation | the minimum allowable temperature of an electrochemical device during a charging process |
| subclassOf | MiniumumTemperature |
MinimumDischargingTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2a1de79f_e927_45a2_9619_3799a0d61e9b
| elucidation | the minimum allowable temperature of an electrochemical device during a discharging process |
| subclassOf | MiniumumTemperature |
MinimumStoichiometricCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_86324806_4263_4d80_b5af_1a7be844ab5b
| elucidation | The minimum allowed value for stoichiometric coefficient for an entity in a chemical substance |
| subclassOf | StoichiometricCoefficient |
MinimumStorageTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ddfd57a_d338_4690_be45_b26884ed6302
| elucidation | the miniumum temperature at which an electrochemical device shall be stored |
| subclassOf | MiniumumTemperature |
MiniumumConcentration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a9873707_8103_4bb4_9e51_83db1e89b1bd
| elucidation | the minimum amount concentration of a species in a phase, either imposed or naturally occuring |
| subclassOf | ConcentrationLimit |
MiniumumTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4747c51d_86ab_4684_a4fb_b05f5c405ea3
| elucidation | the minimum allowable temperature |
| newareLabel | Min-T(C) |
| subclassOf | TemperatureLimit |
| subclasses | MinimumChargingTemperature, MinimumDischargingTemperature, MinimumStorageTemperature |
MolarElectrochemicalPotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7fe804b8_6126_4132_be8f_b4985d61b1f6
| elucidation | electrochemical potential per mole. |
| iupacReference | https://goldbook.iupac.org/terms/view/E01945 |
| restrictions | |
| subclassOf | ISQDerivedQuantity, ElectrochemicalThermodynamicQuantity |
MonoblocContainer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e94f6d42_35e4_4f9f_bb74_5e0628bbd454
| elucidation | case with multiple separate cell compartments |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-18 |
| subclassOf | PrismaticCase |
Mudrib#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1a2eb6db_c927_4039_aea0_8dfab060fd27
| elucidation | ridges in the bottom of the case which support the plate pack (electrode stack) and thus create a space which allows the active material detached from the plates (electrodes) to settle without causing a short-circuit between the plates (electrodes) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-18 |
| subclassOf | Component |
NPRatio#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_05012606_b93d_4016_bbc7_8a927efdf723
| altLabel | ACRatio; AToCRatio; NToPRatio |
| elucidation | quotient of the capacity of the negative electrode and the capacity of the positive electrode in a cell |
| subclassOf | RatioQuantity, ElectrochemicalQuantity |
NearNeutralElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dc205ac2_314e_415c_a2b6_b12e8359d54c
| altLabel | NearNeutralAqueousElectrolyte; NearNeutralSolution |
| elucidation | an aqueous electrolyte with a nominal pH value between 4 and 10. |
| subclassOf | AqueousElectrolyte |
| subclasses | AmmoniumChlorideSolution, Seawater, ZincChlorideSolution, LeclancheElectrolyte |
Note
this covers the pH domain typically described as "weakly acidic", "neutral", and "weakly alkaline"
NegativeElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c94c041b_8ea6_43e7_85cc_d2bce7785b4c
| altLabel | NegativePlate |
| elucidation | for a device having two electrodes, that electrode having the lower electric potential |
| wikidataReference | https://www.wikidata.org/wiki/Q120907506 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=151-13-04; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-05 |
| subclassOf | Electrode |
Note
by convention, an oxidation reaction occurs during discharge
Warning
The term "anode" is often used interchangably with "negative electrode" even though it only applies while the cell is being discharged. When the cell is being charged the negatve electrode becomes the cathode.
NegativeElectrodeActivationEnergyOfReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fda9539d_5232_471c_8945_b9a8ec7247fe
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Reaction rate constant activation energy [J.mol-1]'] |
| subclassOf | ActivationEnergy |
NegativeElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC0#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_21da0fe9_9fb6_4840_a12f_fbcc1ba84fb3
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Minimum stoichiometry'] |
| cidemodKey | ['negative_electrode','active_materials',0,'stoichiometry0','value'] |
| subclassOf | StoichiometricCoefficientAtSOC0 |
NegativeElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC100#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8c336ae9_1818_4b08_a660_4bb83b28351f
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Maximum stoichiometry'] |
| cidemodKey | ['negative_electrode','active_materials',0,'stoichiometry1','value'] |
| subclassOf | StoichiometricCoefficientAtSOC100 |
NegativeElectrodeActiveMaterialMaximumGuestConcentration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e808a26a_5812_49e9_894c_b793c7fe0c38
| elucidation | maximum concentration of lithium in the negative electrode |
| bpxKey | ['Parameterisation','Negative electrode','Maximum concentration [mol.m-3]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'maximum_concentration','value'] |
| subclassOf | MaximumConcentration |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
NegativeElectrodeActiveMaterialOpenCircuitVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0e2f4fe6_570a_4d13_81e9_de1d4f9987af
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','OCP [V]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'OCP','value'] |
| subclassOf | OpenCircuitVoltage |
NegativeElectrodeActiveMaterialParticleRadius#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bfe553c2_a63e_49b6_a209_0855dfc39724
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Particle radius [m]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'particle_radius','value'] |
| subclassOf | ParticleRadius |
NegativeElectrodeActiveMaterialVolumetricSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c5f9b91e_a770_4e9b_837e_fa2a76019111
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Surface area per unit volume [m-1]'] |
| subclassOf | VolumetricSurfaceArea |
NegativeElectrodeCoatingPorosity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5cb403c4_4f28_46cb_81c4_21c5c47ef14a
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Porosity'] |
| cidemodKey | ['negative_electrode','porosity','value'] |
| subclassOf | Porosity |
NegativeElectrodeCoatingThickness#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cdc91ec0_9fc5_4551_bbd9_6824c2920124
| elucidation | thickness of the negative electrode coating |
| bpxKey | ['Parameterisation','Negative electrode','Thickness [m]'] |
| cidemodKey | ['negative_electrode','thickness','value'] |
| subclassOf | CoatingThickness |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
NegativeElectrodeElectronicConductivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_be3da3e2_58a9_4e58_adc2_7336d312717c
| elucidation | the electronic conductivity of the negative electrode |
| bpxKey | ['Parameterisation','Negative electrode','Conductivity [S.m-1]'] |
| cidemodKey | ['negative_electrode','electronic_conductivity','value'] |
| subclassOf | ElectronicConductivity |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
NegativeElectrodeEntropicChangeCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eac57b09_5cc9_41d7_b2c8_40218d7fd47c
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Entropic change coefficient [V.K-1]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'entropy_coefficient','value'] |
| subclassOf | TemperatureCoefficientOfTheOpenCircuitVoltage |
NegativeElectrodeReactionRateConstant#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c5dcb1a2_f2cf_421a_b8ae_47a88a61fce3
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Negative electrode','Reaction rate constant [mol.m-2.s-1]'] |
| cidemodKey | ['negative_electrode','active_materials',0,'kinetic_constant','value'] |
| subclassOf | ReactionRateConstant |
NegativeTerminal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b851c7e9_60bf_4d3d_abe1_8d08d3d85124
| elucidation | accessible conductive part provided for the connection of an external electric circuit to the negative electrode of the cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-24 |
| restrictions | |
| subclassOf | Terminal |
NernstEinsteinEquation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9d7e5fea_a49a_4a19_a8de_8e24c60e420c
| elucidation | an equation relating the limiting molar conductivity Λ_m^0 to the ionic diffusion coefficients. |
| wikidataReference | https://www.wikidata.org/wiki/Q9325636 |
| wikipediaReference | https://en.wikipedia.org/wiki/Einstein_relation_(kinetic_theory)#Nernst%E2%80%93Einstein_equation |
| subclassOf | ElectrochemicalRelation |
Note
Lambda_m^0 = (F^2/(R*T)) * (v_{+} * z_{+}^2 * D_{+} + v_{–} * z_{–}^2 * D_{–})
NernstEquation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fe3a6c9a_85b8_4da6_aa4f_71c8de74939e
| elucidation | An equation that relates the reduction potential of an electrochemical reaction (half-cell or full cell reaction) to the standard electrode potential, temperature, and activities (often approximated by concentrations) of the chemical species undergoing reduction and oxidation. |
| wikidataReference | https://www.wikidata.org/wiki/Q751124 |
| wikipediaReference | https://en.wikipedia.org/wiki/Nernst_equation |
| dbpediaReference | https://dbpedia.org/page/Nernst_equation |
| subclassOf | ElectrochemicalRelation |
Note
E_{eq} = E^0 - (R*T/(z*F))*ln(Q)
Note
An expression of the Law of Mass Action.
NetCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_786a8649_425f_4a2d_b6c8_8a36501b6d49
| elucidation | the net electric charge accumulated in an electrochemical device over time, starting from zero and calculated as the time integral of current with sign preserved, such that charging increases the value and discharging decreases it |
| subclassOf | Capacity |
NetFaradaicCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_14577b99_a8a9_4358_9bc5_ab8c401dd34b
| elucidation | algebraic sum of faradaic currents flowing through an electrode |
| subclassOf | FaradaicCurrent |
Neutralisation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0471ec77_91bc_4a78_be31_b6af613e5966
| altLabel | Neutralization |
| elucidation | insertion or removal of electrons into or from ions to obtain particles having zero electric charge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-01-11 |
| subclassOf | NaturalProcess |
NickelBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0d2aaaf4_1a8a_4a32_abd8_7d0fdf0ae9d2
| elucidation | an electrode in which the primary active material consists of nickel or nickel compounds |
| subclassOf | ActiveElectrode |
| subclasses | LithiumNickelManganeseCobaltOxideElectrode, NickelOxideHydroxideElectrode, SodiumNickelPhosphateElectrode, LithiumNickelCobaltAluminumOxideElectrode, NickelOxideElectrode, LithiumNickelOxideElectrode, LithiumNickelManganeseOxideElectrode |
Note
often represented in IEC cell designations by the letter N
Note
this class is intended to enable designations based on IEC recommendations
NickelOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_76e461aa_5948_4a68_8337_284d11e0fd7d
| altLabel | NiOElectrode |
| elucidation | electrode in which the active material is nickel oxide |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
NickelOxideHydroxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_344ed3a6_481a_499f_afef_631f1cece9ef
| altLabel | NiOOHElectrode; NickelOxyhydroxideElectrode |
| elucidation | an electrode in which the primary active material consists of nickel oxide hydroxide or nickel oxide hydroxide compounds. |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
Note
the combination with zinc metal and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code Z
NominalCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8abde9d0_84f6_4b4f_a87e_86028a397100
| elucidation | the rated capacity that is representative of the typical value associated with a cell as stated by the manufacturer |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | RatedCapacity |
Note
The value for 'nominal capacity' is provided by manufacturers as an indication of the expected performance of the device under a set of defined nominal conditions. It serves as a benchmark for comparing different devices.
Tip
To ensure meaningful comparisons, nominal capacity values should always be evaluated under matching test conditions.
Caution
While nominal capacity provides a useful reference, actual capacity depends on various factors, including temperature, rate, and state-of-health.
NominalVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_639b844a_e801_436b_985d_28926129ead6
| elucidation | suitable approximate value of the voltage used to designate or identify a cell, a battery or an electrochemical system |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-31 |
| subclassOf | Voltage |
Note
Nominal voltage is the standardized voltage value assigned to a battery cell or module based on typical operating conditions. It serves as a reference point for battery specifications but does not represent the actual voltage during operation. Actual cell voltage may vary due to factors such as state-of-charge, temperature, and load conditions.
Caution
Nominal voltage may not accurately reflect voltage under extreme loads or temperatures—always check manufacturer specifications.
NonAqueousElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5f9a9411_05f9_4576_acd3_81d7d41cfe98
| altLabel | NonAqueousElectrolyticSolution; NonAqueousSolution |
| elucidation | an ion-transport medium that does not contain water. |
| subclassOf | LiquidElectrolyte |
| subclasses | IonicLiquidElectrolyte, NonAqueousInorganicElectrolyte |
NonAqueousInorganicElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_addf91e1_ba8c_4a3e_b1e9_092e3e1cc2b3
| elucidation | an electrolyte solution that contains no water and no organic species |
| subclassOf | NonAqueousElectrolyte |
NonPolarizableElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9f466223_e20a_474d_ac4d_6d4b6131c275
| elucidation | an electrode that holds its potential essentially constant by efficiently allowing electric current to pass |
| subclassOf | Electrode |
| subclasses | ReferenceElectrode |
Note
this is a desirable characteristic for a reference electrode
NonSpillableCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c610d869_cc03_44f0_b40d_ca86e945b9c9
| elucidation | cell from which electrolyte cannot escape regardless of its orientation |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-16 |
| subclassOf | ElectrochemicalDevice |
Note
some vented cells or batteries are designed so as to be non-spillable when operated within the limits specified by the manufacturer
NormalHydrogenElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_83ee23b3_2f5c_4afa_b972_ac85e91d7306
| altLabel | NHE |
| elucidation | potential of a platinum electrode in 1 M acid solution. |
| wikidataReference | https://www.wikidata.org/wiki/Q115694592 |
| subclassOf | ReferenceElectrode |
Note
hydrogen gas at 1 atm pressures is bubbled through the acid solution
NumberOfCellsConnectedInParallel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_482173dc_7779_4f12_982c_b19f2cda2dac
| elucidation | the number of electrochemical cells connected in parallel |
| subclassOf | NumberOfEntities, ElectrochemicalQuantity |
NumberOfCellsConnectedInSeries#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9d6a52ed_a53d_4327_a391_f173677a4b1d
| elucidation | the number of electrochemical cells connected in series |
| subclassOf | NumberOfEntities, ElectrochemicalQuantity |
NumberOfElectronsTransferred#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_abfadc99_6e43_4d37_9b04_7fc5b0f327ae
| altLabel | ChargeNumber; ElectronNumberOfAnElectrochemicalReaction |
| elucidation | number of electrons transferred in a charge transfer reaction between an electrode and a single entity (ion, radical-ion, or molecule) of an electroactive substance, whose identity must be specified |
| iupacReference | https://goldbook.iupac.org/terms/view/C00995 |
| subclassOf | PureNumberQuantity, ElectrochemicalKineticQuantity |
NumberOfFiniteVolumeCells#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_47608fd0_cc0d_457e_9141_051935029e3a
| elucidation | number of discrete finite volume cells in a grid or sub-grid |
| subclassOf | NumberOfEntities |
| subclasses | NumberOfFiniteVolumeCellsInZ, NumberOfFiniteVolumeCellsInY, NumberOfFiniteVolumeCellsInX |
NumberOfFiniteVolumeCellsInX#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e73586eb_8475_4879_a43e_087c702ea6bf
| elucidation | number of discrete finite volume cells in a grid or sub-grid in the X-direction |
| subclassOf | NumberOfFiniteVolumeCells |
NumberOfFiniteVolumeCellsInY#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c2a85d02_c6b4_42df_b5ed_a9904ec34a5c
| elucidation | number of discrete finite volume cells in a grid or sub-grid in the Y-direction |
| subclassOf | NumberOfFiniteVolumeCells |
NumberOfFiniteVolumeCellsInZ#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a5e00e3a_736c_4200_8d04_ed222fe07037
| elucidation | number of discrete finite volume cells in a grid or sub-grid in the Z-direction |
| subclassOf | NumberOfFiniteVolumeCells |
NumberOfIterations#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_88dd2bce_fb17_4705_905d_892681812290
| elucidation | the number of times an iterative process should occur |
| subclassOf | PureNumberQuantity |
NyquistPlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a2b01d05_b472_4cf5_b388_e0914db56324
| altLabel | ComplexPlanePlot; NyquistDiagram |
| elucidation | plots of the out of phase vs. the in phase component of the impedance for all the frequencies tested in an impedimetry measurement |
| wikidataReference | https://www.wikidata.org/wiki/Q1333343 |
| wikipediaReference | https://en.wikipedia.org/wiki/Nyquist_stability_criterion#Nyquist_plot |
| subclassOf | ElectrochemicalPlot |
OhmicOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_03a6ce70_5085_4683_bb4e_fc3c18f7143a
| altLabel | OhmicOvervoltage; OhmicPolarization |
| elucidation | part of the electrode polarization arising from an electric current through an ohmic resistance within the electrode or the electrolyte |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-09 |
| subclassOf | Overpotential |
OpenCellFoam#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7cef0da2_8de3_418e_b390_cce9a5d3c129
| altLabel | ReticulatedFoam |
| elucidation | a lightweight, porous material characterized by a network of interconnected pores, typically made from metals or polymers, which allows for high gas and liquid permeability and is used in applications such as filtration, thermal insulation, and energy absorption |
| wikidataReference | https://www.wikidata.org/wiki/Q1312510 |
| wikipediaReference | https://en.wikipedia.org/wiki/Reticulated_foam |
| subclassOf | ManufacturedMaterial |
OpenCircuitVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9c657fdc_b9d3_4964_907c_f9a6e8c5f52b
| altLabel | OCP; OCV; OpenCircuitPotential |
| elucidation | electrode potential of working electrode relative to the reference electrode when no potential or electric current is being applied to the electrochemical cell |
| wikidataReference | https://www.wikidata.org/wiki/Q1812203 |
| wikipediaReference | https://en.wikipedia.org/wiki/Open-circuit_voltage |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-32 |
| subclassOf | ElectrochemicalThermodynamicQuantity, CellVoltage |
| subclasses | EquilibriumElectrodePotential, NegativeElectrodeActiveMaterialOpenCircuitVoltage, PositiveElectrodeActiveMaterialOpenCircuitVoltage |
OrganicElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_564d31be_91cb_4a8f_8369_2a55f1180499
| elucidation | an electrolyte solution with an organic solvent |
| subclassOf | ElectrolyteSolution |
| subclasses | LF100, LP47, LP50, LP57, LP100, LP30, LP40, LP71 |
OutputCable#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_76df6e7b_fc3b_4a1f_98b1_0ca9c0539e4c
| elucidation | cable used for the electrical connection of the battery terminals and the load and/or the charger |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-36 |
| subclassOf | Component |
Overcharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9ee6e728_e8f5_4b36_a045_d63da69dfc85
| elucidation | continued charging of a fully charged secondary cell or battery |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-44 |
| subclassOf | Charging |
Note
overcharge is also the act of charging beyond a certain limit specified by the manufacturer
Overpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1cd1d777_e67b_47eb_81f1_edac35d9f2c6
| altLabel | ElectrodePolarization; Overvoltage; PolarizationPotential |
| elucidation | electrode potential (E) minus the equilibrium electrode potential (Eeq) of an electrochemical reaction |
| wikipediaReference | https://en.wikipedia.org/wiki/Overpotential |
| iupacReference | https://goldbook.iupac.org/terms/view/O04358 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-02 |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
| subclasses | OhmicOverpotential, ReactionOverpotential, ConcentrationOverpotential, SurfaceOverpotential, ActivationOverpotential |
Note
Overpotential is often divided into different contributions: activation overpotential, ohmic overpotential, and concentration overpotential.
Note
Overpotential is the extra voltage required to drive an electrochemical reaction beyond its thermodynamic equilibrium potential. It arises due to kinetic barriers such as activation energy, mass transport limitations, and charge transfer resistance. Overpotential is a key factor in battery efficiency, electrolysis, and corrosion studies, as it affects reaction rates and energy losses.
Oxidation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c6a35be8_7ce2_415b_be9e_3a5ff9a7f2ad
| altLabel | OxidationReaction |
| elucidation | chemical reaction in which electrons are transferred from a substance to oxygen to form a compound of that substance or, more generally, in which substance loses electrons |
| iupacReference | https://goldbook.iupac.org/terms/view/O04362 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=113-02-15 |
| subclassOf | RedoxReaction |
| subclasses | AnodicReaction |
Note
Electrons are generally transferred to another substance by a reduction reaction.
OxygenElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e2c91edd_dd01_4309_9735_6fe5280261d4
| elucidation | a gas diffusion electrode in which the active material is oxygen (O2) |
| subclassOf | GasDiffusionElectrode |
Note
the combination with zinc metal and an alkali metal hydroxide electrolyte is designated using IEC electrochemical system letter code P
P12103310#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_27f95593_47f1_4494_b6bb_ac0733df185d
| altLabel | P12/103/310 |
| elucidation | a prismatic case with a nominal length of 12 mm, width of 103 mm, and height of 310 mm |
| subclassOf | PrismaticCase |
P1212080#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c54fbb75_0528_49c0_97e0_258798a947e4
| altLabel | P12/120/80 |
| elucidation | a prismatic case with a nominal length of 12 mm, width of 120 mm, and height of 80 mm |
| subclassOf | PrismaticCase |
P1212081#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d1199193_b203_4fcb_971c_ccf6332973ae
| altLabel | HEV2; P12/120/81 |
| elucidation | a prismatic case with a nominal length of 12 mm, width of 120 mm, and height of 81 mm |
| subclassOf | PrismaticCase |
P1212085#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4ca551d2_2969_4c18_96ce_db99831ed0af
| altLabel | P12/120/85 |
| elucidation | a prismatic case with a nominal length of 12 mm, width of 120 mm, and height of 85 mm |
| subclassOf | PrismaticCase |
P1312085#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_93358d9b_8976_42d0_84ae_e5a2400c3ea7
| altLabel | HEV1; P13/120/85 |
| elucidation | a prismatic case with a nominal length of 13 mm, width of 120 mm, and height of 85 mm |
| subclassOf | PrismaticCase |
P1714891#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_56def0d5_fe57_40d7_85e3_97872fa2a3e1
| altLabel | P17/148/91; PHEV2 |
| elucidation | a prismatic case with a nominal length of 17 mm, width of 148 mm, and height of 91 mm |
| subclassOf | PrismaticCase |
P1865140#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_289da03d_606a_4668_a195_e81627bc80b7
| altLabel | P18/65/140 |
| elucidation | a prismatic case with a nominal length of 18 mm, width of 65 mm, and height of 140 mm |
| subclassOf | PrismaticCase |
P1868107#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d1bf3cdd_d618_40e3_b055_a952ef632157
| altLabel | P18/68/107 |
| elucidation | a prismatic case with a nominal length of 18 mm, width of 68 mm, and height of 107 mm |
| subclassOf | PrismaticCase |
P20100141#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bba6b94e_c0d7_40bf_a9d6_413ff458397c
| altLabel | P20/100/141 |
| elucidation | a prismatic case with a nominal length of 20 mm, width of 100 mm, and height of 141 mm |
| subclassOf | PrismaticCase |
P21148128#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0e366263_fded_4509_bddd_392d839013e4
| altLabel | P21/148/128 |
| elucidation | a prismatic case with a nominal length of 21 mm, width of 148 mm, and height of 128 mm |
| subclassOf | PrismaticCase |
P2117385#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3b714a22_4212_4f4f_b578_140c357b18de
| altLabel | P21/173/85; PHEV1 |
| elucidation | a prismatic case with a nominal length of 21 mm, width of 173 mm, and height of 85 mm |
| subclassOf | PrismaticCase |
P27148129#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_619c2e56_077d_4bb4_9062_65fb75763651
| altLabel | P27/148/129 |
| elucidation | a prismatic case with a nominal length of 27 mm, width of 148 mm, and height of 129 mm |
| subclassOf | PrismaticCase |
P2714891#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f4a6a682_fd7c_43df_bed2_df70848d6026
| altLabel | P27/148/91 |
| elucidation | a prismatic case with a nominal length of 27 mm, width of 148 mm, and height of 91 mm |
| subclassOf | PrismaticCase |
P2714898#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_50d9652c_6b68_457c_bd72_f89a8e549bcf
| altLabel | P27/148/98 |
| elucidation | a prismatic case with a nominal length of 27 mm, width of 148 mm, and height of 98 mm |
| subclassOf | PrismaticCase |
P2770102#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a5c1b56f_5c82_4e3b_800e_5a280331348b
| altLabel | P27/70/102 |
| elucidation | a prismatic case with a nominal length of 27 mm, width of 70 mm, and height of 102 mm |
| subclassOf | PrismaticCase |
P2770120#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7018bd4e_6ca1_44eb_a558_6585c0bf8547
| altLabel | P27/70/120 |
| elucidation | a prismatic case with a nominal length of 27 mm, width of 70 mm, and height of 120 mm |
| subclassOf | PrismaticCase |
P2770131#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_afe918c7_0634_40c1_9421_78ea2fe707e3
| altLabel | P27/70/131 |
| elucidation | a prismatic case with a nominal length of 27 mm, width of 70 mm, and height of 131 mm |
| subclassOf | PrismaticCase |
P29135214#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_37504d4d_2aff_4f7f_9f46_a24e258028e8
| altLabel | P29/135/214 |
| elucidation | a prismatic case with a nominal length of 29 mm, width of 135 mm, and height of 214 mm |
| subclassOf | PrismaticCase |
P32173115#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8112c7ba_e58a_463b_8661_b836bb6d7c86
| altLabel | BEV1; P32/173/115 |
| elucidation | a prismatic case with a nominal length of 32 mm, width of 173 mm, and height of 115 mm |
| subclassOf | PrismaticCase |
P32173225#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_948234f6_4095_4419_af30_00ab297a7694
| altLabel | BEV3; P32/173/225 |
| elucidation | a prismatic case with a nominal length of 32 mm, width of 173 mm, and height of 225 |
| subclassOf | PrismaticCase |
P4014895#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_41ab11ec_af9f_401d_b80d_474c1dc86cd6
| altLabel | P40/148/95 |
| elucidation | a prismatic case with a nominal length of 40 mm, width of 148 mm, and height of 95 mm |
| subclassOf | PrismaticCase |
P45173115#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_36444184_1100_4271_869f_7f2d983aaecb
| altLabel | BEV2; P45/173/115 |
| elucidation | a prismatic case with a nominal length of 45 mm, width of 173 mm, and height of 115 mm |
| subclassOf | PrismaticCase |
P45173225#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2c63d7f4_09ba_4ca9_baa2_a1da899bf477
| altLabel | BEV4; P45/173/225 |
| elucidation | a prismatic case with a nominal length of 45 mm, width of 173 mm, and height of 225 mm |
| subclassOf | PrismaticCase |
PRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c4120ce7_f1ff_4917_b59e_002454a65040
| elucidation | an indicator of the electric power at which an electrochemical device is operated |
| subclassOf | RatioQuantity, ElectrochemicalQuantity |
| subclasses | ChargingPRate, DischargingPRate |
PRateUnit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2e9ccb34_6da6_4715_89ba_90913f436b41
| elucidation | the class of units for describing the C-Rate in electrochemistry and related domains |
| restrictions |
|
| subclassOf | SIDimensionalUnit |
| subclasses | WattPerWattHour |
PalladiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f9f056bb_a38b_43bd_a6bd_99d618431f4d
| elucidation | an electrode in which the primary active material consists of palladium or palladium compounds |
| subclassOf | ActiveElectrode |
ParallelConnection#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_efc4f7ab_850d_443c_a17f_184983021f96
| elucidation | arrangement of cells or batteries wherein all the positive terminals and all the negative terminals, respectively, are connected together. |
| wikidataReference | https://www.wikidata.org/wiki/Q302489 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-39 |
| subclassOf | Assembled |
ParallelSeriesConnection#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d5da9948_e95b_4f12_a2d2_10a48f390c52
| elucidation | arrangement of cells or batteries wherein parallel connected cells or batteries are connected in series. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-40 |
| subclassOf | Assembled |
ParasiticReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b79d4f9e_5727_4895_8d7f_5fc18d83eb90
| elucidation | an unwanted side reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-07; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-13 |
| subclassOf | SideReaction |
Note
in a galvanic cell, parasitic reactions may cause inefficiencies or loss of performance
Note
Parasitic reactions are a subset of side reactions specifically associated with unwanted charge transfer.
Particle#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e13b7945_65ac_476b_85ee_5cdc59f5c919
| elucidation | a small localized object which can be described by several physical or chemical properties, such as volume, density, or mass. |
| wikipediaReference | https://en.wikipedia.org/wiki/Particle |
| subclassOf | ElectrochemicalComponent |
ParticleCracking#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f6341d7d_7620_48f5_97b2_99b55c349169
| elucidation | a degradation mechanism in electrochemical cells in which fractures in the particles degrade performance |
| restrictions | |
| subclassOf | ElectrochemicalDegradationPhenomenon |
Note
Often afflicts active material particles.
ParticleRadius#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b92e382f_5109_4f60_ab5e_c89d340419a9
| elucidation | radius of a particle |
| subclassOf | Radius, ElectrochemicalQuantity |
| subclasses | PositiveElectrodeActiveMaterialParticleRadius, NegativeElectrodeActiveMaterialParticleRadius |
PassivationLayer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d16eec87_c646_4566_bda6_7d3357cda061
| elucidation | an interfacial layer of an electrode surface, that causes the electrode to become passivated |
| subclassOf | InterfacialRegion |
| subclasses | SolidElectrolyteInterphase |
PastedPlate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a1ec9e3c_c624_4848_af13_89a6bc54d77c
| altLabel | PastedElectrode |
| elucidation | plate in which the active material is applied as a paste to a conductive current collector |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-03 |
| subclassOf | CoatedElectrode |
PeakCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_382b10dc_83aa_4e77_a1d5_1edd06fd1e05
| elucidation | in dynamic voltammetric techniques, the maximum value of the faradaic current attained by varying the applied potential in the current-potential or I-E curve |
| iupacReference | https://goldbook.iupac.org/terms/view/P04457 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
Example
Typical examples of imposed potential programmes in dynamic voltammetric techniques resulting in peak-shaped responses are linear-scan voltammetry, cyclic voltammetry, ac voltammetry, differential pulse voltammetry, square-wave voltammetry, stripping voltammetry, and derivative techniques.
PeakPotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e4a221e0_afc9_4464_91d4_e0c8ecdf79e8
| elucidation | electrode potential of the working electrode at which the peak current is attained |
| iupacReference | https://doi.org/10.1351/goldbook.P04464 |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
PercentQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cf386c1e_e62b_43ef_9b0e_e5e58935d63f
| elucidation | a quantity that is expressed as a percentage |
| restrictions | |
| subclassOf | RatioQuantity |
| subclasses | WeightPercent, VolumePercent |
PercentUnit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8430be19_6cae_4a15_a570_3c15d0d190eb
| elucidation | unit of percentage |
| subclassOf | FractionUnit |
PerforatedFoil#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c84a1d8f_e405_42ba_8c80_bbe3b9c6c66a
| elucidation | a thin sheet that has been punched with a pattern of holes, offering controlled porosity and strength for uses in filtration, sound suppression, and structural components |
| subclassOf | ManufacturedMaterial |
PhosphoricAcidSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_78f75a64_55b6_4243_a35e_3d279c83209b
| elucidation | a solution of phosphoric acid (H3PO4) dissolved in water (H2O) |
| subclassOf | AcidicElectrolyte |
PhotoelectrolyticCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7760b241_775f_4be1_b827_59f9bde9e5b2
| elucidation | electrolytic cell in which a chemical reaction is influenced by the absorption of light |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-04-19 |
| subclassOf | ElectrolyticCell |
PlateGroup#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_457a8f92_0a19_4773_8114_a42edff32248
| elucidation | assembly of plates of the same polarity electrically connected together |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-04 |
| restrictions | |
| subclassOf | Assembled |
PlatinumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d8a9a88e_d437_4fef_bc3c_65a1fe627061
| elucidation | an electrode in which the primary active material consists of platinum or platinum compounds |
| subclassOf | ActiveElectrode |
| subclasses | PlatinumElectrode |
PlatinumElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2d32a81a_2148_41bd_84fb_467aa8de4a8f
| altLabel | PtElectrode |
| elucidation | foil, wire, disc, or mesh electrode made of platinum, which is the most commonly used metallic working electrode in electrochemistry |
| wikidataReference | https://www.wikidata.org/wiki/Q20851363 |
| restrictions | |
| subclassOf | PlatinumBasedElectrode |
Note
Pt has a very small overpotential for hydrogen evolution, which determines the negative potential limit in protic solvents. Pt adsorbs hydrogen, resulting in hydrogen adsorption/desorption waves. However, the positive potential limit in polar aprotic solvents free of oxygen and water is higher than that for any other commonly used electrode materials. In the presence of water and/or oxygen, Pt forms a film of oxide (and/or chemisorbed oxygen), resulting in potentially interfering waves or peaks.
Polarity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_16a5de33_a2ca_4563_80d4_6caeb08d97ca
| elucidation | nominal property of an electrode, having values negative or positive according to the sign of the electrode potential, or neutral when the electrode potential is zero. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-14 |
| subclassOf | ElectrochemicalProperty |
PolarityReversal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_83d2c2d4_ffa9_42f4_9264_a0c59c657607
| altLabel | CellReversal |
| elucidation | reversing of the polarities of the electrodes of a cell, generally due to over-discharge of a low-capacity cell in a series arrangement |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-03 |
| subclassOf | IntentionalAgency |
PolarizableElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c2024587_3237_474e_8df9_91d10db2df47
| elucidation | electrode whose potential changes with an applied potential |
| subclassOf | Electrode |
PolymerElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c6d6c0a1_a06b_437a_978f_a469d3071ca9
| elucidation | a polymer matrix capable of ion conduction |
| wikidataReference | https://www.wikidata.org/wiki/Q107223477 |
| wikipediaReference | https://en.wikipedia.org/wiki/Polymer_electrolytes |
| subclassOf | Electrolyte |
PorousElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3663991d_9319_4f7a_922b_f0e428b58801
| elucidation | porous electrodes consist of porous matrices of a single reactive electronic conductor or a mixture of solids that include essentially non-conducting, reactive materials in addition to electronic conductors. An electrolytic solution fills the void spaces of the porous matrix. |
| restrictions |
|
| subclassOf | Electrode |
| subclasses | GasDiffusionElectrode |
PorousSeparator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_58413d4e_2885_459e_ac06_8d45e661cf91
| elucidation | a separator with a porosity greater than 0 |
| subclassOf | Separator |
PositiveElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_aff732a9_238a_4734_977c_b2ba202af126
| altLabel | PositivePlate |
| elucidation | for a device having two electrodes, that electrode having the higher electric potential |
| wikidataReference | https://www.wikidata.org/wiki/Q120907518 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=151-13-05; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-06 |
| subclassOf | Electrode |
| subclasses | TubularPlate |
Note
by convention, a reduction reaction occurs during discharge
Warning
The term "cathode" is often used interchangably with "positive electrode" even though it only applies while the cell is being discharged. When the cell is being charged the positive electrode becomes the anode.
PositiveElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC0#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_80920875_62ac_4e29_b970_ec4316e76aa5
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Minimum stoichiometry'] |
| cidemodKey | ['positive_electrode','active_materials',0,'stoichiometry0','value'] |
| subclassOf | StoichiometricCoefficientAtSOC0 |
PositiveElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC100#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_99041897_5c08_40ed_9118_3e77e9b0e191
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Maximum stoichiometry'] |
| cidemodKey | ['positive_electrode','active_materials',0,'stoichiometry1','value'] |
| subclassOf | StoichiometricCoefficientAtSOC100 |
PositiveElectrodeActiveMaterialMaximumGuestConcentration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c69a9d55_823f_4113_a305_ebc89dde7de3
| elucidation | maximum concentration of lithium in the positive electrode |
| bpxKey | ['Parameterisation','Positive electrode','Maximum concentration [mol.m-3]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'maximum_concentration','value'] |
| subclassOf | MaximumConcentration |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
PositiveElectrodeActiveMaterialOpenCircuitVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_52ab4fdd_f945_4541_9ce6_cd6fd3a05861
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','OCP [V]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'OCP','value'] |
| subclassOf | OpenCircuitVoltage |
PositiveElectrodeActiveMaterialParticleRadius#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_58400817_3282_46e5_942e_3a1538631403
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Particle radius [m]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'particle_radius','value'] |
| subclassOf | ParticleRadius |
PositiveElectrodeActiveMaterialVolumetricSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a1e73c5_e91b_4365_88d4_1e1f476bf776
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Surface area per unit volume [m-1]'] |
| subclassOf | VolumetricSurfaceArea |
PositiveElectrodeCoatingPorosity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7481c4c9_c247_4248_a045_a1077230acba
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Porosity'] |
| cidemodKey | ['positive_electrode','porosity','value'] |
| subclassOf | Porosity |
PositiveElectrodeCoatingThickness#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_62f5beeb_6d1e_442a_8048_3ebe08882964
| elucidation | thickness of the positive electrode coating |
| bpxKey | ['Parameterisation','Positive electrode','Thickness [m]'] |
| cidemodKey | ['positive_electrode','thickness','value'] |
| subclassOf | CoatingThickness |
Note
this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases.
PositiveElectrodeElectronicConductivity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_43f77743_1af6_4a0f_9cc6_285c2a450549
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Conductivity [S.m-1]'] |
| cidemodKey | ['positive_electrode','electronic_conductivity','value'] |
| subclassOf | ElectronicConductivity |
PositiveElectrodeEntropicChangeCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9d558b56_d3b8_429a_a4e2_d2ffab895e42
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Entropic change coefficient [V.K-1]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'entropy_coefficient','value'] |
| subclassOf | TemperatureCoefficientOfTheOpenCircuitVoltage |
PositiveElectrodeReactionRateConstant#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_404126e0_cb1b_44e4_98dc_2474185767a1
| elucidation | this is a very specific term with a narrow conceptualization, used mostly as an anchor term for supporting interoperability; a more general term is suitable in most cases. |
| bpxKey | ['Parameterisation','Positive electrode','Reaction rate constant [mol.m-2.s-1]'] |
| cidemodKey | ['positive_electrode','active_materials',0,'kinetic_constant','value'] |
| subclassOf | ReactionRateConstant |
PositiveTerminal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_522f0c0b_cf33_4328_af71_12e38dae6798
| elucidation | accessible conductive part provided for the connection of an external electric circuit to the positive electrode of the cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-25 |
| restrictions | |
| subclassOf | Terminal |
PostiveElectrodeActivationEnergyOfReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_56b9cd1f_5397_4385_9292_30d93d9e7a05
| elucidation | the activation energy barrier in an Arrhenius expression for the positive electrode charge transfer reaction |
| bpxKey | ['Parameterisation','Positive electrode','Reaction rate constant activation energy [J.mol-1]'] |
| subclassOf | ActivationEnergy |
PotassiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0afe79ed_dc0d_4b3e_88fa_ae0c7b1e88b5
| elucidation | an electrode in which the primary active material consists of potassium or potassium compounds |
| subclassOf | ActiveElectrode |
PotassiumHydroxideSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_26a0dc36_8171_4a84_88dd_0f5dd7cb2d20
| altLabel | AqueousPotassiumHydroxideSolution; KOHSolution |
| elucidation | a solution of potassium hydroxide (KOH) dissolved in water (H2O) |
| subclassOf | AlkalineElectrolyte |
PotassiumInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c183f697_8995_477c_9ccd_5c12d98e3633
| elucidation | an insertion electrode in which the guest molecule is potassium |
| subclassOf | InsertionElectrode |
PotentialPulse#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2dd44ff6_425a_4377_b86e_fa2bd567819f
| elucidation | Rapid, transient change in the amplitude of an electric potential, from a baseline value to a higer or lower value, followed by a rapid return to the baseline value. |
| subclassOf | ElectricPotentialSignal |
PotentialScanRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_264f40d1_17c9_4bc7_9c47_5cfb18132278
| altLabel | PotentialSweepRate; ScanRate; SweepRate |
| elucidation | the rate of change of applied potential with time |
| restrictions | |
| subclassOf | ISQDerivedQuantity, ElectrochemicalControlQuantity |
PotentialTimePlot#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cd7d24a5_db31_4d76_97d9_367c809f099e
| altLabel | ElectricPotentialTimeCurve; ElectricPotentialTimePlot; PotentialTimeCurve |
| elucidation | plot of the time-dependence of instantaneous electric potential following a change in applied current |
| subclassOf | ElectrochemicalPlot |
PotentiometricSelectivityCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_136744ff_0563_4df7_aa03_4219d70392a0
| altLabel | SelectivityCoefficient; SelectivityConstant; SelectivityFactor |
| elucidation | parameter defining the ability of an ion-selective electrode to distinguish a particular ion, i.e., a primary ion A from an interfering ion B |
| iupacReference | https://doi.org/10.1351/goldbook.P04791 |
| subclassOf | ElectrochemicalQuantity |
PotentiometricStrippingAnalysis#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_916b1863_f417_4b94_9407_9d749ada9ed5
| altLabel | PSA |
| elucidation | two-step electrochemical measurement in which 1) material is accumulated at an electrode and 2) the material is removed by chemical reaction or electrochemically at constant current with measurement of electrode potential |
| subclassOf | CurrentControlledProcess |
Note
historically for the analysis of metal ions, mercury ions were added to the test solution to form a mercury amalgam when reduced. Alternatively, an HMDE or MFE was used and the oxidizing agent added after amalgam formation. However, the toxicity of mercury and its compounds have all but precluded the present-day use of mercury
Note
the accumulation is similar to that used in stripping voltammetry
Note
the stripping potentiogram shows staircase curves of potential as a function of time. Frequently, the first derivative is displayed (dE/dt=f(t)), as this produces peak-shaped signals. The time between transitions (peaks) is proportional to the concentration of analyte in the test solution
Note
the time between changes in potential in step 2 is related to the concentration of analyte in the solution
Potentiostat#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a9fc3f77_e48e_4bce_b118_044d608722f6
| elucidation | measuring instrument for electric current that controls the potential difference between a working electrode and a reference electrode and measures the electric current between a working electrode and an auxiliary electrode. |
| wikipediaReference | https://en.wikipedia.org/wiki/Potentiostat |
| subclassOf | Device |
| subclasses | VSP300, HCP1005, VMP3e, SP150e, SP300, SP50e, HCP803, VSP, VSP3e, SP200, VMP300 |
PotentiostaticCycling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5a5ef74b_e4a7_4b85_ab21_ae0febebaf58
| elucidation | electrochemical method where the voltage of an electrochemical device is held constant while measuring the current response over multiple charge-discharge cycles to evaluate its performance and stability |
| restrictions | |
| subclassOf | VoltageControlledProcess, Cycling |
Pouch#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_af751d07_f0b7_43c7_911d_cba864189b2b
| elucidation | a form factor describing a pouch cell, which has the shape of a rectangular prism where the thickness less than the length and the width and the case material is flexible |
| subclassOf | FormFactor |
PouchCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_74459386_875c_4303_b774_60125b599d06
| elucidation | a soft pouch case that is described by its length, width, and height |
| subclassOf | Case |
Note
often represented in cell designations by the letter P
PouchCellElectrolyteFilling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dbc73810_d334_40b8_b73a_7ad2c0b3615f
| elucidation | the process of filling an electrolyte in a pouch cell |
| bvco | https://bvco.ontology.link/BVCO_0c42fa38_503b_4fe4_90a6_c27730627514 |
| subclassOf | ElectrolyteFilling |
PouchCellPackaging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d5e99e1f_049c_4111_b97f_613eb652492a
| elucidation | the process of inserting a dry electrode stack into a pouch cell container |
| subclassOf | CellPackaging |
Powder#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ee479886_6805_4018_95e1_500185e44215
| elucidation | a material comprised of many particles |
| restrictions | |
| subclassOf | ManufacturedMaterial |
PowerControlledProcess#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dc1af38d_438e_40c2_8a68_0d631a8f46f2
| elucidation | a process in which the electric power is controlled |
| subclassOf | Process |
| subclasses | PowerHold |
PowerDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a7eb870c_4ef7_4ccd_85e8_4b7b726d7a2a
| altLabel | VolumetricPowerDensity |
| elucidation | the quotient of the power of an energy-storage device or system and its volume |
| subclassOf | ElectrochemicalPerformanceQuantity |
PowerHold#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_87340877_e8ed_4061_ac2f_cb1c121e7193
| altLabel | ConstantPowerOperation; FonctionnementAPuissanceConstante |
| elucidation | a process in which the electric power is kept constant |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=485-11-02 |
| subclassOf | PowerControlledProcess |
| subclasses | ConstantPowerCharging, ConstantPowerDischarging |
PressureReliefVent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f330680b_347e_45b3_9113_5ce09d18d60b
| elucidation | vent of special design which allows to release gas from a cell in order to avoid excessive internal pressure and thereby to preclude rupture or explosion of the cell container |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-41 |
| subclassOf | Component |
Prismatic#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6775b232_c58a_4303_8164_137261329db6
| elucidation | a form factor describing a prismatic cell, which has the shape of a rectangular prism |
| subclassOf | FormFactor |
Prismatic#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_83fbc038_0c2e_4d04_91d7_cdf30f8a5535
| elucidation | [a sign that] qualifies a cell or a battery having the shape of a parallelepiped whose faces are rectangular |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-38 |
| subclassOf | ElectrochemicalProperty |
PrismaticCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_43cd6e14_dd43_41b5_b5b4_344d53841603
| elucidation | a rigid prismatic case that is described by characteristic length, width, and height |
| restrictions |
|
| subclassOf | Case |
| subclasses | electrochemistry.P1312085, electrochemistry.P4014895, electrochemistry.P2714898, electrochemistry.P45173225, electrochemistry.P21148128, electrochemistry.P1212081, electrochemistry.P45173115, electrochemistry.P32173115, electrochemistry.P27148129, electrochemistry.P12103310, electrochemistry.P32173225, electrochemistry.P1865140, electrochemistry.P20100141, electrochemistry.P1212085, electrochemistry.P2117385, electrochemistry.P1212080, MonoblocContainer, electrochemistry.P1868107, electrochemistry.P29135214, electrochemistry.P2770131, electrochemistry.P1714891, electrochemistry.P2714891, electrochemistry.P2770102, electrochemistry.P2770120 |
Note
often represented in cell designations by the letter P
PrismaticCellElectrolyteFilling#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_99a4c4b9_90d1_41ef_9073_894d9b35e751
| elucidation | the process of filling an electrolyte in a prismatic cell |
| bvco | https://bvco.ontology.link/BVCO_b5540155_6bab_4d81_bf3d_41c171d63a0f |
| subclassOf | ElectrolyteFilling |
PrismaticCellPackaging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1a532a1a_a48c_4b08_854c_4f6c212c8b59
| elucidation | the process of inserting a dry jelly roll into a prismatic cell container |
| subclassOf | CellPackaging |
ProtonInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_11a774f3_d20a_4741_bd81_cae53230bd0c
| elucidation | an insertion electrode in which the guest molecule is a proton (hydrogen) |
| subclassOf | InsertionElectrode |
PseudoOpenCircuitVoltageData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a49d9cd1_bec8_4d1d_9657_d47983e9135d
| altLabel | POCVData; PseudoOCVData |
| elucidation | a dataset resulting from a pseudo-open-circuit voltage experiment or simulation |
| subclassOf | TimeSeriesElectricalDataSet |
PseudoOpenCircuitVoltageTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_00244072_8d24_4e34_9f6a_c7b2b132b2c8
| elucidation | a test where an electrochemical device is operated at a very low current, approximating equilibrium behaviour, in order to extract a pseudo-open-circuit-voltage profile |
| subclassOf | ElectrochemicalTesting |
PulseCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8372fdaa_dc01_4abf_9b75_442d0b28f263
| elucidation | charging an electrochemical device at a given charging current for a defined short duration |
| restrictions | |
| subclassOf | ConstantCurrentCharging, CurrentPulsing |
PulseCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_346519a4_006c_496d_8f36_74e38814ed2d
| altLabel | MagnitudeOfCurrentPulse; PulseMagnitudeCurrent |
| elucidation | the magnitude of a current pulse applied to an electrochemical cell during pulsed potentiometry and related techniques |
| subclassOf | ElectricCurrent, ElectrochemicalControlQuantity |
| subclasses | MaximumPulseDischargingCurrent |
PulseDischarging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_acdedc56_61da_40ca_aa81_db4c06d82dd5
| elucidation | discharging an electrochemical device at a given charging current for a defined short duration |
| restrictions | |
| subclassOf | ConstantCurrentDischarging, CurrentPulsing |
PulseDuration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3cd0e74e_292c_48ed_a831_4cb8753d9a56
| elucidation | time interval during which the excitation signal deviates from the base line in normal pulse voltamme- try, differential pulse voltammetry, and related techniques |
| iupacReference | https://doi.org/10.1351/goldbook.P04947 |
| subclassOf | Duration, ElectrochemicalControlQuantity |
| subclasses | MaximumDischargingPulseDuration |
PulseMagnitude#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_762ba55f_9b56_4c31_865f_cff2e7d0a94b
| elucidation | a single value, for instance a mean, root mean square or peak value characterizing the aggregate instantaneous values of a unidirectional pulse with respect to the common initial and final value |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=702-03-03 |
| subclassOf | ObjectiveProperty |
PulseResponseData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b7f05e67_d7e8_469f_971f_0cb4d4c7e857
| elucidation | time series data that contains a controlled pulse and the response of a system |
| subclassOf | TimeSeriesElectricalDataSet |
PulseVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4d2b102b_3515_4591_b079_69232c44f9dc
| altLabel | MagnitudeOfPotentialPulse; PulseMagnitudePotential |
| elucidation | The magnitude of a voltage pulse applied to an electrochemical cell during pulsed amperometry and related techniques. |
| subclassOf | CellVoltage, ElectrochemicalControlQuantity |
QuarterTransitionTimePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2705525b_7512_48bf_825b_f2d0409bede4
| elucidation | electric potential of the indicator electrode, in chronopotentiometry at constant current, at the instant when the time that has elapsed since the application of current is equal to one-fourth of the transition time |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
R1025#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e73cb0b7_e2af_4c1a_b05b_503df25a8bf5
| elucidation | a coin case with a nominal diameter of 10.0 mm and a height of 2.5 mm |
| subclassOf | CoinCase |
R11108#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dac5067c_e6f2_4fca_a915_5b7b918ca1c4
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 10.8 mm |
| subclassOf | CoinCase |
R1121#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_31b7ac6a_b1eb_45e7_a075_b6063080c949
| altLabel | R55 |
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 2.1 mm |
| subclassOf | CoinCase |
R1126#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cf6eebea_24f6_48d2_8389_65eba0393762
| altLabel | R56 |
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 2.6 mm |
| subclassOf | CoinCase |
R1130#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_39c6f5a0_5dc8_4112_b432_b9fece568ca2
| elucidation | a coin case with a nominal diameter of 11.5 mm and a height of 3.0 mm |
| subclassOf | CoinCase |
R1131#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b442217f_02f6_4991_aadf_22d81369223c
| altLabel | R54 |
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 3.1 mm |
| subclassOf | CoinCase |
R1136#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_21eef598_0799_4cb1_b2d5_6e072a8579a3
| altLabel | R42 |
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 3.6 mm |
| subclassOf | CoinCase |
R1142#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_abffe8cd_14d3_4437_84e6_5dfc61b057e8
| altLabel | R43 |
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 4.2 mm |
| subclassOf | CoinCase |
R1154#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fe054d02_41c7_40e9_8206_3440ab6a67b2
| altLabel | R44 |
| elucidation | a coin case with a nominal diameter of 11.6 mm and a height of 5.4 mm |
| subclassOf | CoinCase |
R1216#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_36e05f2f_d279_4816_b5ae_6bb4cef02736
| elucidation | a coin case with a nominal diameter of 12.5 mm and a height of 1.6 mm |
| subclassOf | CoinCase |
R1220#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_296b95b0_c4cb_45db_a8ca_f638379870c4
| elucidation | a coin case with a nominal diameter of 12.5 mm and a height of 2.0 mm |
| subclassOf | CoinCase |
R1225#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f7e253da_b12e_40b0_ba51_8cb039839ab6
| elucidation | a coin case with a nominal diameter of 12.05 mm and a height of 2.5 mm |
| subclassOf | CoinCase |
R1511#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ce4352a7_f0b1_4f27_9184_6deebf8bbe96
| altLabel | R52 |
| elucidation | a coin case with a nominal diameter of 15.8 mm and a height of 11.1 mm |
| subclassOf | CoinCase |
R1616#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d5f67a0c_ed56_479d_9b68_6003142f98b0
| elucidation | a coin case with a nominal diameter of 16.0 mm and a height of 1.6 mm |
| subclassOf | CoinCase |
R1632#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f2cf71c1_f2d7_4131_82e0_2701cdecea55
| elucidation | a coin case with a nominal diameter of 16.0 mm and a height of 3.2 mm |
| subclassOf | CoinCase |
R18650#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3d2f9945_2319_4a08_899b_6a5ffabd4925
| altLabel | R18/65; R1865 |
| elucidation | a cylindrical case with a nominal diameter of 18 mm and height of 65 mm |
| subclassOf | CylindricalCase |
R19660#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9dc236d9_c962_4fcc_94db_911a25241472
| altLabel | R19/66 |
| elucidation | a cylindrical case with a nominal diameter of 19 mm and height of 66 mm |
| subclassOf | CylindricalCase |
R2012#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9c0e683b_40d7_4786_b31f_910dd68b2ea8
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 1.2 mm |
| subclassOf | CoinCase |
R2016#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_30c8fb1b_3c75_49f5_9395_c15dfaf71b12
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 1.6 mm |
| subclassOf | CoinCase |
Note
often used in wrist watches
R2020#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9017b8b9_e21e_4961_a3c1_c0aeef3fe795
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 2.0 mm |
| subclassOf | CoinCase |
R2025#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9ceffffd_b918_48c2_aad9_5db16fa6149d
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 2.5 mm |
| subclassOf | CoinCase |
Note
often used in wrist watches and automobile remotes
R2032#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_94497aca_52a0_48e3_9b76_157b050e35b3
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 3.2 mm |
| subclassOf | CoinCase |
Note
very common case for lithim metal cells
R2040#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fab4bd12_c4ed_417a_92a3_bcb109000d82
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 4.0 mm |
| subclassOf | CoinCase |
R2050#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_400cb3e0_27b5_4d9a_be80_f86ad2757763
| elucidation | a coin case with a nominal diameter of 20.0 mm and a height of 5.0 mm |
| subclassOf | CoinCase |
R21700#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3b048a62_8591_4bf1_8fac_3d647beb9323
| altLabel | R21/70; R2170 |
| elucidation | a cylindrical case with a nominal diameter of 21 mm and height of 70 mm |
| subclassOf | CylindricalCase |
R2320#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fbf40756_3265_4468_bcdb_88d162afc539
| elucidation | a coin case with a nominal diameter of 23.0 mm and a height of 2.0 mm |
| subclassOf | CoinCase |
R2325#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0689e6fc_cda7_4e04_b1eb_41459a0a4736
| altLabel | R23/3 |
| elucidation | a coin cell case with a nominal diameter of 23.0 mm and a height of 2.5 mm |
| subclassOf | CoinCase |
R2330#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_11579b47_cbf8_45ac_aed3_343ea533b346
| elucidation | a coin case with a nominal diameter of 23.0 mm and a height of 3.0 mm |
| subclassOf | CoinCase |
R2335#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_885ffc4d_2b29_42d6_8597_6fc879c21d60
| elucidation | a coin case with a nominal diameter of 23.0 mm and a height of 3.5 mm |
| subclassOf | CoinCase |
R2354#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_af92a3ae_e870_4676_9ae7_cda277b1e6e1
| elucidation | a coin case with a nominal diameter of 23.0 mm and a height of 5.4 mm |
| subclassOf | CoinCase |
R2412#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a4e7c3d6_45bd_402a_962f_5d4b88af84ff
| elucidation | a coin case with a nominal diameter of 24.5 mm and a height of 1.2 mm |
| subclassOf | CoinCase |
R2430#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_11e9f765_4fea_456b_bd8e_4624b9c752ae
| elucidation | a coin case with a nominal diameter of 24.5 mm and a height of 3.0 mm |
| subclassOf | CoinCase |
R2450#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_aaafc5d6_050a_4c51_b1ca_db5839aad4de
| elucidation | a coin case with a nominal diameter of 24.5 mm and a height of 5.0 mm |
| subclassOf | CoinCase |
R2477#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a87d4b9d_ba91_4549_aed4_508d1160c0c0
| elucidation | a coin case with a nominal diameter of 24.5 mm and a height of 7.7 mm |
| subclassOf | CoinCase |
R26650#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_95d841eb_2002_4411_9f8e_927d6c60e4b3
| altLabel | R26/65 |
| elucidation | a cylindrical case with a nominal diameter of 26 mm and height of 65 mm |
| subclassOf | CylindricalCase |
R26700#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f2251de5_0a7d_4887_8bd1_c906db84abee
| altLabel | R26/70 |
| elucidation | a cylindrical case with a nominal diameter of 26 mm and height of 70 mm |
| subclassOf | CylindricalCase |
R27660#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6ee40e33_ccbf_410e_a8fa_047a9eae0362
| altLabel | R27/66 |
| elucidation | a cylindrical case with a nominal diameter of 27 mm and height of 66 mm |
| subclassOf | CylindricalCase |
R3032#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_99475d6c_304c_4d6b_9eca_fbc42f768be5
| elucidation | a coin case with a nominal diameter of 30.0 mm and a height of 3.2 mm |
| subclassOf | CoinCase |
R32134#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c066bf32_ed62_4a11_bf4f_25fe6e8e68b5
| altLabel | R32/134 |
| elucidation | a cylindrical case with a nominal diameter of 32 mm and height of 134 mm |
| subclassOf | CylindricalCase |
R32700#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ad9cdad6_66e8_471e_a7c6_b79f37c20814
| altLabel | R32/70 |
| elucidation | a cylindrical case with a nominal diameter of 32 mm and height of 70 mm |
| subclassOf | CylindricalCase |
R38136#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f58476a9_f008_4502_ac94_2f1ebbce1fe3
| altLabel | R38/136 |
| elucidation | a cylindrical case with a nominal diameter of 38 mm and height of 136 mm |
| subclassOf | CylindricalCase |
R38138#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_169fb6e4_cbed_4b00_ace0_8731123a35b4
| altLabel | R38/138 |
| elucidation | a cylindrical case with a nominal diameter of 38 mm and height of 138 mm |
| subclassOf | CylindricalCase |
R40108#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_56177336_3ae5_48cb_93ca_a8c73216ea73
| altLabel | R40/108 |
| elucidation | a cylindrical case with a nominal diameter of 40 mm and height of 108 mm |
| subclassOf | CylindricalCase |
R40920#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6eaa3c0d_e1f3_4c6e_91a6_9b8c5a21b8cb
| altLabel | R40/92 |
| elucidation | a cylindrical case with a nominal diameter of 40 mm and height of 92 mm |
| subclassOf | CylindricalCase |
R416#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_027d437e_3bbf_4eda_940b_e509f8d2b993
| elucidation | a coin case with a nominal diameter of 4.8 mm and a height of 1.6 mm |
| subclassOf | CoinCase |
R46800#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1afa2c7e_6ddd_4a9b_9623_40975131a304
| altLabel | R46/80; R4680 |
| elucidation | a cylindrical case with a nominal diameter of 46 mm and height of 80 mm |
| subclassOf | CylindricalCase |
R512#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_94209cd8_f68f_40e6_ac70_cfb97a16e593
| elucidation | a coin case with a nominal diameter of 5.8 mm and a height of 1.3 mm |
| subclassOf | CoinCase |
R516#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_76318e8e_c1ba_49ac_8029_a951bd9dc955
| altLabel | R62 |
| elucidation | a coin case with a nominal diameter of 5.8 mm and a height of 1.6 mm |
| subclassOf | CoinCase |
R521#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a5038d4_24af_4ea8_8d56_a156faeb98e2
| altLabel | R63 |
| elucidation | a coin case with a nominal diameter of 5.8 mm and a height of 2.1 mm |
| subclassOf | CoinCase |
R527#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b56a95e6_eea5_4a58_99aa_045fd28e75d3
| altLabel | R64 |
| elucidation | a coin case with a nominal diameter of 5.8 mm and a height of 2.7 mm |
| subclassOf | CoinCase |
R54137#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_69e59b3d_b19d_4f8e_8b9c_84eb64307626
| altLabel | R54/137 |
| elucidation | a cylindrical case with a nominal diameter of 54 mm and height of 137 mm |
| subclassOf | CylindricalCase |
R54215#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fc9e7ba3_5084_4c2a_b488_c6a96af99232
| altLabel | R54/215 |
| elucidation | a cylindrical case with a nominal diameter of 54 mm and height of 215 mm |
| subclassOf | CylindricalCase |
R616#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dc019f3f_e5f3_4eeb_bab9_a845a02223a0
| altLabel | R65 |
| elucidation | a coin case with a nominal diameter of 6.8 mm and a height of 1.65 mm |
| subclassOf | CoinCase |
R621#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_15ec346a_6d3c_4161_95e1_9cf889965f85
| altLabel | R60 |
| elucidation | a coin case with a nominal diameter of 6.8 mm and a height of 2.1 mm |
| subclassOf | CoinCase |
R626#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6b275583_433f_46f7_aafd_ebc9409257cc
| altLabel | R66 |
| elucidation | a coin case with a nominal diameter of 6.8 mm and a height of 2.6 mm |
| subclassOf | CoinCase |
Note
often used in wrist watches
R626#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_68c1c36e_1548_4247_bae2_fe7102a2c4ff
| altLabel | R66 |
| elucidation | a coin case with a nominal diameter of 6.8 mm and a height of 2.6 mm |
| subclassOf | CoinCase |
R712#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bb2faf17_b819_4876_b17f_fa82917cf85d
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 1.3 mm |
| subclassOf | CoinCase |
R716#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_34cb7ea7_2436_46e0_806e_a3c7835ae228
| altLabel | R67 |
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 1.65 mm |
| subclassOf | CoinCase |
R721#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3279f575_1c62_4bf2_a6e8_de4c7ff36f8e
| altLabel | R58 |
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 2.1 mm |
| subclassOf | CoinCase |
R726#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_06db7d9e_d224_4360_b926_734f0349d396
| altLabel | R59 |
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 2.6 mm |
| subclassOf | CoinCase |
R731#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_88100e2c_0f6e_4483_afbf_30029a6702c6
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 3.1 mm |
| subclassOf | CoinCase |
R736#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0c09df01_b251_4f75_b6e3_2578ba1a10c9
| altLabel | R41 |
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 3.6 mm |
| subclassOf | CoinCase |
R754#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_028069a2_4e03_409e_8af2_4c8df32b5c5e
| altLabel | R48 |
| elucidation | a coin case with a nominal diameter of 7.9 mm and a height of 5.4 mm |
| subclassOf | CoinCase |
R9#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e0869e7a_36fe_4e33_9843_a5dc19fcb488
| elucidation | a coin case with a nominal diameter of 15.5 mm and a height of 6.0 mm |
| subclassOf | CoinCase |
R916#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ebf314c7_81ad_4d77_9da4_b454520fda0e
| altLabel | R68 |
| elucidation | a coin case with a nominal diameter of 9.5 mm and a height of 1.6 mm |
| subclassOf | CoinCase |
R921#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eb1c9aa3_ad4f_4f2a_80f6_d6c8a8bc0d9a
| altLabel | R69 |
| elucidation | a coin case with a nominal diameter of 9.5 mm and a height of 2.1 mm |
| subclassOf | CoinCase |
R926#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a277e420_2483_44d5_a2bc_4e421dea231a
| altLabel | R57 |
| elucidation | a coin case with a nominal diameter of 9.5 mm and a height of 2.6 mm |
| subclassOf | CoinCase |
R927#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_387e1e59_e511_4acb_b5ec_fa7360ec7557
| elucidation | a coin case with a nominal diameter of 9.5 mm and a height of 2.7 mm |
| subclassOf | CoinCase |
R932#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9a23f61b_fef0_40f8_84f9_2057f90f6a95
| elucidation | a coin case with a nominal diameter of 9.3 mm and a height of 3.2 mm |
| subclassOf | CoinCase |
Note
8 of these in series are used to form an A23 battery
Note
rarely used independently
R936#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d67a0921_15e4_4c73_804e_97b2d66452b8
| altLabel | R45 |
| elucidation | a coin case with a nominal diameter of 9.5 mm and a height of 3.6 mm |
| subclassOf | CoinCase |
RatedCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9b3b4668_0795_4a35_9965_2af383497a26
| elucidation | capacity value of a battery [or electrode or electrochemical device] determined under specified conditions and declared by the manufacturer |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-15 |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | Capacity |
| subclasses | TheoreticalCapacity, MaximumCapacity, MinimumCapacity, NominalCapacity, RetainedCapacity, RecoveredCapacity |
Note
The term 'rated capacity' is used by manufacturers to specify the energy storage capability of a product under some controlled test conditions. The value is often determined during a constant current load until the a voltage limit is reached, under controlled temperature conditions. The 'rated capacity' helps to compare performance across different devices and provides a bisis for performing system engineering calculations. It can also be used as a basis for calculating other terms like the C-Rate, state-of-charge, and state-of-health of the device. The rated capacity at beginning of life serves as a reference for capacity-fade analysis during long-term cycling.
Tip
The value of rated capacity should be reported together with information on the conditions under which it was determined (e.g. voltage limits, applied current, temperature).
Caution
The value for rated capacity does not necessarily represent the actual capacity in real-world usage, as factors like temperature, rate, and state-of-health affect the effective capacity.
RatedEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_da215d1a_196a_465f_9de8_89f6fbb76982
| elucidation | the amount of energy that an electrochemical device can store under some specific conditions |
| subclassOf | StoredEnergy |
| subclasses | MaximumStorageEnergy |
ReactionOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f4fadc4e_ca7d_4e4e_89cf_eacf45b4041e
| altLabel | ReactionOvervoltage; ReactionPolarization |
| elucidation | part of the electrode polarization arising from a chemical reaction impeding the electrode reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-10 |
| subclassOf | Overpotential |
| subclasses | AnodicOverpotential, CathodicOverpotential, CrystalizationOverpotential |
ReactionRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e2514bf9_d012_4990_b68b_6f37443f18f6
| elucidation | rate at which a chemical reaction takes place |
| wikidataReference | https://www.wikidata.org/wiki/Q3394849 |
| wikipediaReference | https://en.wikipedia.org/wiki/Reaction_rate |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-02 |
| subclassOf | ElectrochemicalKineticQuantity |
ReactionRateConstant#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0335e3f6_d1d8_4daa_8376_a9285f1bc9f1
| elucidation | a proportionality constant which quantifies the rate and direction of a chemical reaction by relating it with the concentration of reactants |
| wikidataReference | https://www.wikidata.org/wiki/Q658700 |
| wikipediaReference | https://en.wikipedia.org/wiki/Reaction_rate_constant |
| subclassOf | ISQDerivedQuantity, ElectrochemicalKineticQuantity |
| subclasses | NegativeElectrodeReactionRateConstant, PositiveElectrodeReactionRateConstant |
RealElectricImpedance#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_abf986d2_90d5_4746_b42b_89dc7cc1bf0f
| altLabel | ACResistance |
| elucidation | the imaginary part of the complex electric impedance |
| wikidataReference | https://www.wikidata.org/wiki/Q1048490 |
| subclassOf | ElectricImpedance |
Record#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_59c041fc_eaa1_40fc_9b3e_1a6aca6119fd
| elucidation | a measurement of one or more quantities, usually at a given time |
| subclassOf | Dataset |
Record#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_838a927f_775e_4c6d_8e39_7865548608c2
| elucidation | an entry in a dataset, typically consisting of one line. |
| hiddenLabel | Rec# |
| subclassOf | ElectrochemicalQuantity |
RecoveredCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_73f169de_d189_4d7c_893f_a2549771825c
| altLabel | RecoveryCapacity |
| elucidation | the capacity that can be obtained from an electrochemical device after it has been subjected to some specified conditions, then recharged and discharged according to a recommended protocol |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | RatedCapacity |
RectangularElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_14377ecb_5ead_431e_831e_159d622bd0ea
| elucidation | an electrode in the shape of a rectangle |
| restrictions |
|
| subclassOf | Electrode |
RedoxReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2d366f85_a644_4b3e_a641_fce81e72d158
| altLabel | Redox |
| elucidation | a chemical reaction involving a change in oxidation state of one or more atomic centers through electron transfer or an equivalent formal process |
| wikidataReference | https://www.wikidata.org/wiki/Q82682 |
| wikipediaReference | https://en.wikipedia.org/wiki/Redox |
| subclassOf | ChemicalReaction |
| subclasses | Oxidation, Reduction, ElectrochemicalReaction |
Note
Redox reactions include both electrochemical and non-electrochemical processes; when the electron transfer occurs across an electrode–electrolyte interface the reaction is electrochemical.
Reduction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_109a38d4_daaa_4748_a803_044fb7c97673
| altLabel | ReductionReaction |
| elucidation | chemical reaction in which oxygen is removed from an oxide, implying a transfer of electrons from the oxygen atoms, or, more generally, in which a substance gains electrons |
| iupacReference | https://goldbook.iupac.org/terms/view/R05222 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=113-02-16 |
| subclassOf | RedoxReaction |
| subclasses | CathodicReaction |
Note
Electrons are generally transferred from another substance by an oxidation reaction.
ReferenceElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7729c34e_1ae9_403d_b933_1765885e7f29
| elucidation | Electrode with a well-defined and constant equilibrium potential with respect to which it is possible to measure or calculate electrode potentials of other electrodes by including them in an appropriate electrochemical cell. |
| wikidataReference | https://www.wikidata.org/wiki/Q653954 |
| wikipediaReference | https://en.wikipedia.org/wiki/Reference_electrode |
| iupacReference | https://goldbook.iupac.org/terms/view/R05229 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-15 |
| subclassOf | NonPolarizableElectrode |
| subclasses | StandardHydrogenElectrode, SilverChlorideElectrode, NormalHydrogenElectrode, ReversibleHydrogenElectrode, SaturatedCalomelElectrode |
Note
Electrode that maintains an essentially constant potential under the conditions prevailing in an electrochemical measurement, and that serves for observation, measurement, or control of the potential of the indicator or working electrode.
Note
In potentiometry, under zero current condition, the essentially constant potential is achieved by ensuring a constant composition of solution in contact with the electrode forming a half-cell. Practical reference half-cells are generally non-polarizable electrodes of the 2nd kind (metal | insoluble salt | ion), constructed so that their electrolyte solutions serve as salt bridges to the solutions under investigation.
Note
“Double” junction reference electrodes are recommended when the reference electrolyte contains interfering components.
Example
The standard hydrogen electrode represents the primary standard in electrochemistry. Elec- trodes of the 2nd kind, such as Ag | AgCl, Hg | Hg2, Cl2, Hg | Hg2SO4, and Hg | HgO, can be used as reference electrodes in aqueous solutions containing ions Cl^{−}, SO_4^{2−}, and OH^{−}, respectively.
ReferencePerformanceTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c7e37e88_ed86_4acd_99ee_b6a2a5fcbd24
| elucidation | a periodic assessment of battery degradation during life testing |
| subclassOf | Electrochemical |
ReferenceThermodynamicTemperature#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_db40df7e_1aa5_49a7_85cb_2aa2c1c70489
| elucidation | the thermodynamic temperature that is the reference for a calculation |
| subclassOf | ThermodynamicTemperature |
ResidualActiveMass#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_526bf81a_0572_49ff_a8cc_85efc343c1c2
| elucidation | charged active material remaining in a cell following a discharge to a specified end-of-discharge voltage. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-37 |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
ResidualCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d47985f1_6bd7_4c4f_894f_13a6cab38bb5
| elucidation | electric charge capacity remaining in a cell or battery following a discharge, operation or storage under specific test conditions |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-16 |
| subclassOf | Capacity |
ResidualCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_07e219c3_890f_488f_bd96_bee8e445d764
| altLabel | BackgroundCurrent |
| elucidation | Electric current that flows, at a particular value of the applied potential, in the absence of the substance whose electrode behaviour is being investigated, i.e. a "blank" solution. |
| iupacReference | https://goldbook.iupac.org/terms/view/R05311 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
ResponseTimeAtAnISE#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1a104474_c326_4a29_ac26_26a05ac8f72c
| elucidation | Duration between the time when an ion-selective electrode and an external reference electrode (the two completing the ion-selective electrode cell) are brought into contact with a sample solution (or the time at which the activity of the ion of interest in solution is changed) and the first time at which the slope of the cell potential vs. time plot (ΔE/Δt) becomes equal to a limiting value selected on the basis of the experimental conditions and/or requirements concerning accuracy. |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
Resting#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_35469eeb_7fc3_4317_a4b5_b3be7015444b
| elucidation | a step in an electrochemical testing workflow in which the electrochemical device is held at open-circuit conditions (i.e. cell current = 0 A) and occuring in series with at least one active (i.e. cell current ~= 0 A) step. |
| restrictions | |
| subclassOf | OpenCircuitHold, CurrentHold |
RestingTime#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2678a656_4a27_4706_8dde_b0a93e9b92fa
| altLabel | RestingDuration |
| elucidation | the duration during which an electrochemical device is kept at open-circuit conditions |
| subclassOf | Duration, ElectrochemicalControlQuantity |
RetainedCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b8d4042d_329a_450f_9088_2fc0e16a8667
| elucidation | the capacity that an electrochemical device can deliver after being stored under some specified conditions |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | RatedCapacity |
ReversibleHydrogenElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0d9ba00d_04bc_4bdc_85af_3380694f6f68
| elucidation | a practical hydrogen electrode whose potential depends on the pH of the solution |
| wikidataReference | https://www.wikidata.org/wiki/Q900576 |
| wikipediaReference | https://en.wikipedia.org/wiki/Reversible_hydrogen_electrode |
| subclassOf | ReferenceElectrode |
RhodiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_399b10cd_8a2e_47be_96b8_295890bd2460
| elucidation | an electrode in which the primary active material consists of rhodium or rhodium compounds |
| subclassOf | ActiveElectrode |
RotatingDiskElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6c421175_477f_45e0_8b6c_c3464f5351c5
| altLabel | RDE |
| elucidation | A disc electrode that is embedded in the centre of a cylinder which rotates in solution around the longitudinal cylinder axis. |
| wikidataReference | https://www.wikidata.org/wiki/Q3587619 |
| wikipediaReference | https://en.wikipedia.org/wiki/Rotating_disk_electrode |
| subclassOf | RoundElectrode |
| subclasses | RotatingRingDiskElectrode |
Note
The current is directly proportional to the electrode area and the electrode is uniformly accessible.
Note
The limiting current is given by the Levich equation.
RotatingDiskSpeed#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1900143f_cbc0_415f_9057_9382022a7bfe
| elucidation | rotational frequency of a rotating component in a rotating disk electrode |
| subclassOf | RotationalFrequency, ElectrochemicalControlQuantity |
Note
The rotating component can be the main rotating disk or a secondary ring as used in rotating ring disk electrodes.
RotatingRingDiskElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7f4d74cd_d0a5_4908_9da9_7629fe419917
| altLabel | RRDE |
| elucidation | a second annular working electrode positioned concentric with a rotating disc electrode to make a rotating ring-disc electrode (RRDE) |
| wikidataReference | https://www.wikidata.org/wiki/Q5829908 |
| wikipediaReference | https://en.wikipedia.org/wiki/Rotating_ring-disk_electrode |
| restrictions | |
| subclassOf | RotatingDiskElectrode |
Note
This arrangement is largely used in mechanistic studies.
RoundCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eacb141f_6ab4_491f_8603_a3e025cefc82
| elucidation | a case that is described by its diameter and height |
| restrictions |
|
| subclassOf | Case |
| subclasses | CoinCase, CylindricalCase |
Note
often represented in cell designations by the letter R
RoundElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a8bfac4f_3f30_4e6d_8d8e_34b1eeecb614
| elucidation | an electrode in the shape of a circle |
| subclassOf | Electrode |
| subclasses | RotatingDiskElectrode |
RutheniumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7ffe1cb6_f87e_4b4a_8ce7_c98e2a584cb1
| elucidation | an electrode in which the primary active material consists of ruthenium or ruthenium compounds |
| subclassOf | ActiveElectrode |
RutheniumOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_10a91aba_da41_4309_a926_ddc0f285c2c1
| altLabel | RuO2Electrode |
| elucidation | electrode in which the active material is ruthenium oxide |
| subclassOf | MetalOxideElectrode |
SP150e#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5662253f_ce1d_414d_8ca4_1e30b3396334
| altLabel | SP-150e |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
SP200#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ba0fe910_d2b0_4873_a904_fc15c4752f72
| altLabel | SP-200 |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
Note
designed for corrosion with the 100 fA accuracy ultra-low current option
SP300#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2fc80f11_14fb_4961_a2e7_6be270b390c8
| altLabel | SP-300 |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
SP50e#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_72ad8f96_246f_49c2_b498_7dba17ecb297
| altLabel | SP-50e |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
SaltBridge#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_637c576e_a50e_47ae_8c74_2024ce4c6d0f
| elucidation | means of making electrolytic connection between two half cells without introducing a significant liquid junction potential. |
| wikidataReference | https://www.wikidata.org/wiki/Q899119 |
| wikipediaReference | https://en.wikipedia.org/wiki/Salt_bridge |
| dbpediaReference | https://dbpedia.org/page/Salt_bridge |
| subclassOf | ElectrochemicalComponent |
Note
A typical construction is a tube of an inert material (e.g. agar agar) filled with a solution containing an electrolyte with approximately equal ion mobilities of the cation and the anion (e.g., KNO3, KCl), with the ends of the tube immersed in the electrolyte solution of the half cells.
SamplingInterval#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_52ac73c7_763c_4fda_93cd_a2db9dfc2dab
| elucidation | time interval during which the current is measured in pulse voltammetry |
| iupacReference | https://doi.org/10.1351/goldbook.S05465 |
| subclassOf | Duration, ElectrochemicalControlQuantity |
SamplingTime#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ecb6dfdf_bd3d_4339_8a1c_d32abbef30ba
| elucidation | duration of the sampling interval in pulse voltammetry |
| iupacReference | https://doi.org/10.1351/goldbook.S05467 |
| subclassOf | Duration, ElectrochemicalControlQuantity |
SaturatedCalomelElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_82b66bfe_ec25_417b_ba65_b631ddaaca0e
| elucidation | A reference electrode based on the reaction between elemental mercury and mercury(I) chloride. |
| wikidataReference | https://www.wikidata.org/wiki/Q898591 |
| wikipediaReference | https://en.wikipedia.org/wiki/Saturated_calomel_electrode |
| subclassOf | ReferenceElectrode |
SaturatedSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_27a81a51_e8d7_42fb_834f_0d068fa45d89
| elucidation | solution which has the same concentration of a solute as one that is in equilibrium with undissolved solute at specified values of the temperature and pressure |
| iupacReference | https://doi.org/10.1351/goldbook.S05471 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-01-14 |
| subclassOf | Solution |
SealedCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8d3bf304_78e4_4e99_abe1_6ab429e44ed1
| elucidation | cell which remains closed and does not release either gas or liquid when operated within the limits specified by the manufacturer |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-17 |
| subclassOf | ElectrochemicalDevice |
Note
a sealed cell may be equipped with a safety device to prevent a dangerously high internal pressure and is designed to operate during its life in its original sealed state
Seawater#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1c0c8f43_7349_4646_94e3_c36727c5c2e3
| elucidation | a naturally occuring solution of salts (mostly NaCl) dissolved in water |
| subclassOf | NearNeutralElectrolyte |
SelfDischarging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_444992e5_43f8_44a1_9767_b408dbe54330
| elucidation | phenomenon by which a cell or battery loses energy in other ways than by discharge into an external circuit |
| wikidataReference | https://www.wikidata.org/wiki/Q1418367 |
| wikipediaReference | https://en.wikipedia.org/wiki/Self-discharge |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-27 |
| subclassOf | CapacityFade |
Separator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_331e6cca_f260_4bf8_af55_35304fe1bbe0
| altLabel | ElectrochemicalSeparator |
| elucidation | in an electrochemical cell, device made of insulating material permeable to the ions of the electrolyte and prohibiting totally or partially the mixing of the substances on both sides. |
| wikidataReference | https://www.wikidata.org/wiki/Q1718181 |
| wikipediaReference | https://en.wikipedia.org/wiki/Separator_(electricity) |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-11; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-17 |
| restrictions |
|
| subclassOf | ElectrochemicalComponent |
| subclasses | AsymmetricMembrane, PorousSeparator, GlassFibreSeparator, IonExchangeMembrane |
Note
Membranes and diaphragms are special forms of electrochemical separators.
SeparatorPorosity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a4858e4d_dd3b_48ce_97ba_3eeb8571b633
| elucidation | the porosity of a separator |
| bpxKey | ['Parameterisation','Separator','Porosity'] |
| cidemodKey | ['separator','porosity','value'] |
| subclassOf | Porosity |
SeparatorThickness#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_47288277_4aed_447e_b659_0c975d031406
| elucidation | the thickness of a separator |
| bpxKey | ['Parameterisation','Separator','Thickness [m]'] |
| cidemodKey | ['separator','thickness','value'] |
| subclassOf | Thickness |
SeriesConnection#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8c71739c_27c1_4d19_a0ff_02545296af43
| elucidation | arrangement of cells or batteries wherein the positive terminal of each cell or battery is connected to the negative terminal of the next cell or battery in sequence. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-41 |
| subclassOf | Assembled |
SeriesParallelConnection#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bed5b5f9_b89d_45e3_a8c2_81b70ae21847
| elucidation | arrangement of cells or batteries wherein in series connected cells or batteries are connected in parallel. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-42 |
| subclassOf | Assembled |
ShelfLife#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e8ec76bf_2a60_4982_8cde_02dfbd2e626f
| altLabel | StorageLife |
| elucidation | duration, under specific conditions, at the end of which a battery [ or electrochemical device ] has retained the ability to perform a specified function |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-47 |
| subclassOf | ElectrochemicalPerformanceQuantity |
ShortCircuitCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_75c28dc8_3d7d_4b6e_861e_6c8b1ad7d644
| elucidation | maximum current which should be delivered by a cell or battery into an external circuit with zero electric resistance, or an external circuit which depresses the cell or battery voltage to approximately zero volt |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-26 |
| subclassOf | CellCurrent |
SideReaction#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f47611a2_e081_47f0_b287_3fda5f706154
| altLabel | SecondaryReaction |
| elucidation | chemical reaction which occurs in addition to the main process |
| wikidataReference | https://www.wikidata.org/wiki/Q22117434 |
| wikipediaReference | https://en.wikipedia.org/wiki/Side_reaction |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-06; https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-13 |
| subclassOf | ChemicalReaction |
| subclasses | ParasiticReaction |
Caution
Side reactions can lead to degradation, gas formation, or loss of active material.
SignalReferencePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_68059d94_4c21_4065_b329_07faeebc7e87
| altLabel | BaselinePotential |
| elucidation | Potential to which the magnitude of an excitation signal is compared to. |
| restrictions | |
| subclassOf | ElectrochemicalControlQuantity |
SiliconBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_79e12290_d1e5_4c41_916c_18f1e4d7fb51
| elucidation | an electrode in which the primary active material consists of silicon or silicon compounds |
| subclassOf | ActiveElectrode |
SiliconGraphiteElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e8c39ecc_29d1_4172_996e_d5b05dc88015
| elucidation | an electrode with blended silicon and graphite active materials |
| restrictions | |
| subclassOf | BlendedActiveElectrode |
SiliconOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4143b1c2_2d21_419e_af45_247d4c78ce7e
| elucidation | electrode in which the active material is silicon oxide |
| subclassOf | MetalOxideElectrode |
SiliconOxideGraphiteElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2fd5964b_0c65_4413_8b8c_849639e6d1e7
| elucidation | an electrode with blended silicon oxide and graphite active materials |
| restrictions | |
| subclassOf | BlendedActiveElectrode |
SilverBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f897db90_afd6_42e7_8d1f_0fcba856e45a
| elucidation | an electrode in which the primary active material consists of silver or silver compounds |
| subclassOf | ActiveElectrode |
| subclasses | SilverElectrode |
SilverChlorideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6ec59f99_5f26_4a7d_9b90_b52e0f8ad190
| elucidation | A type of reference electrode based on the reaction between sliver and silver chloride. |
| wikidataReference | https://www.wikidata.org/wiki/Q900939 |
| wikipediaReference | https://en.wikipedia.org/wiki/Silver_chloride_electrode |
| subclassOf | ReferenceElectrode |
SilverElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a462859d_d8bd_48ea_8bde_1576f1248a1e
| altLabel | AgElectrode |
| elucidation | electrode in the form of foil, mesh, wire, rod, tube, powder, pellets, or single crystal of silver |
| wikidataReference | https://www.wikidata.org/wiki/Q20851418 |
| restrictions |
|
| subclassOf | SilverBasedElectrode |
Note
Ag electrodes were first used in potentiometry for the determination of Cl−, Br−, I−, as well as for Ag+ ions, to which they are sensitive.
SilverOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a3745142_b97a_4e19_b7ed_03599f56e81d
| elucidation | an electrode in which the primary active material consists of manganese dioxide or vmanganese dioxide compounds |
| restrictions | |
| subclassOf | MetalOxideElectrode |
Note
the combination with zinc metal and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code S
SingleCoatedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_418c59bd_dc9d_438b_bc7c_494fbd1bb4f8
| elucidation | an electrode that is coated on only one side of the current collector |
| illustration |  |
| subclassOf | CoatedElectrode |
SinusoidalCurrentWaveform#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d61deb36_b397_4811_bf7a_66d8e4578c6e
| elucidation | alternating current consistent with a sinusoidal waveform |
| subclassOf | AlternatingCurrent |
SinusoidalPotentialWaveform#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c74bb11c_e875_4112_b9cf_00d0890ef1f5
| elucidation | Sinusoidal alternating potential of small amplitude (10 to 50 mV) of constant frequency (10 Hz to 100 kHz). |
| subclassOf | ElectricPotentialSignal |
Slurry#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_13fad803_de44_490d_a77e_c330c9168087
| elucidation | a semi-liquid mixture, typically composed of particles suspended in a solvent |
| subclassOf | PhaseHeterogeneousMixture |
SodiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_df4dd678_9642_47c9_84dd_4bb09f369f53
| elucidation | an electrode in which the primary active material consists of sodium or sodium compounds |
| subclassOf | ActiveElectrode |
SodiumChromiumOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f8f81b27_1d6c_42d8_a7e9_5a2534102562
| elucidation | electrode in which the active material is sodium chromium oxide |
| restrictions | |
| subclassOf | MetalOxideElectrode |
SodiumCobaltOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ee0278fb_932d_48cd_a20a_c1b89b29d68b
| elucidation | electrode in which the active material is sodium cobalt oxide |
| restrictions | |
| subclassOf | CobaltBasedElectrode, MetalOxideElectrode |
SodiumCobaltPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9e24d403_8531_416e_a9ec_d1ec5508bcb1
| elucidation | electrode in which the active material is sodium cobalt phosphate |
| restrictions | |
| subclassOf | CobaltBasedElectrode, MetalOxideElectrode |
SodiumHydroxideSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ebd01982_6b0c_48e7_90ef_7b7342009449
| altLabel | AqueousSodiumHydroxideSolution; NaOHSolution |
| elucidation | a solution of sodium hydroxide (NaOH) dissolved in water (H2O) |
| subclassOf | AlkalineElectrolyte |
SodiumInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d936c767_1530_419c_93f4_59e08f0d702c
| elucidation | an insertion electrode in which the guest molecule is sodium |
| subclassOf | InsertionElectrode |
SodiumIronHexacyanoferrateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8968eb7a_9a65_4286_a596_c31b998df329
| elucidation | electrode in which the active material is sodium iron hexacyanoferrate |
| subclassOf | MetalOxideElectrode |
SodiumIronPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_502a98a3_ce47_421f_8e0a_016ed171c900
| elucidation | electrode in which the active material is sodium iron phosphate |
| restrictions | |
| subclassOf | IronPhosphateBasedElectrode, MetalOxideElectrode |
SodiumManganeseHexacyanoferrateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4edb47d6_107c_40a2_be7c_6b26e9d296da
| elucidation | electrode in which the active material is sodium manganese hexacyanoferrate |
| subclassOf | ManganeseBasedElectrode, MetalOxideElectrode |
SodiumManganeseOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e8d9e091_a56d_4a24_a305_d1bac55cfce3
| elucidation | electrode in which the active material is sodium manganese oxide |
| restrictions | |
| subclassOf | ManganeseBasedElectrode, MetalOxideElectrode |
SodiumManganesePhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cc0468a2_1851_4d3d_92a6_b4059db0c056
| elucidation | electrode in which the active material is sodium manganese phosphate |
| restrictions | |
| subclassOf | ManganesePhosphateBasedElectrode, MetalOxideElectrode |
SodiumMetatitanateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_103c9e6c_9c26_430a_9fb9_f4f041e970b0
| elucidation | electrode in which the active material is sodium metatitanate |
| subclassOf | TitaniumBasedElectrode, MetalOxideElectrode |
SodiumNickelPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4a13e538_4c08_4ba9_8ebc_98a866208e01
| elucidation | electrode in which the active material is sodium nickel phosphate |
| restrictions | |
| subclassOf | NickelBasedElectrode, MetalOxideElectrode |
SodiumTitaniumOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c5f51531_1452_4654_82e5_0505491c2c7d
| elucidation | electrode in which the active material is sodium titanium oxide |
| subclassOf | TitaniumBasedElectrode, MetalOxideElectrode |
SodiumTitaniumPhosphateElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cff80dd5_19a9_4357_8fb9_410a978153e2
| elucidation | electrode in which the active material is sodium titanium phosphate |
| subclassOf | TitaniumBasedElectrode, MetalOxideElectrode |
SolidAmalgamElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_65c90d8d_9712_4f3f_b830_d8163ec4cfcc
| altLabel | SAE |
| elucidation | electrode made of a solid amalgam of an appropriate metal |
| restrictions |
|
| subclassOf | MetalElectrode |
Note
Liquid and paste amalgams can also be used.
Note
Solid amalgam electrodes are electrochemically similar to the hanging mercury drop electrode. Their main advantages are high hydrogen overpotential (similar to liquid mercury); a wide working potential range; the simple mechanical, chemical, and electrochemical regeneration of the surface; a long lifetime; low toxicity of material (comparable with that of dental amalgams); applicability for field measurements; compatibility with flow-through systems; simple construction, without mobile parts; and easy miniaturization.
SolidElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0508a114_544a_4f54_a7de_9b947fb4b618
| altLabel | FastIonConductor; SolidStateElectrolyte |
| definition | a solid electrolyte is a solid material where the predominant charge carriers are ions. |
| wikipediaReference | https://en.wikipedia.org/wiki/Fast_ion_conductor |
| subclassOf | Electrolyte |
Example
NASICON (Na Super Ionic Conductor), which has the general formula Na1+xZr2P3-xSix O12 , 0 < x < 3.
SolidElectrolyteInterphase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ecf136cb_2584_4cb1_98b7_2d2b3d22e40d
| altLabel | SEI |
| elucidation | an interfacial layer that forms between an electrode phase and and electrolyte phase, due to the decomposition of the electrolyte |
| subclassOf | PassivationLayer |
| subclasses | CathodeElectrolyteInterphase |
Note
although the definition could apply to any electrode, it is conventionally used to describe interfacial layers that form on the anode of an electrochemical cell.
SpaceChargeLayer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e3f6eacc_f661_404e_ac16_45d2fa1f5d89
| altLabel | DepletionLayer; DepletionRegion; DepletionZone; JunctionRegion; SpaceChargeRegion |
| elucidation | an interphase formed when two materials with different chemical potentials are brought in contact with each other, and the atoms or electrons are unable to migrate to establish local charge neutrality |
| wikidataReference | https://www.wikidata.org/wiki/Q288224 |
| wikipediaReference | https://en.wikipedia.org/wiki/Depletion_region |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=521-02-82 |
| subclassOf | InterfacialRegion |
Spacer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_49909cd1_44f4_41b4_877a_82a52845a5cb
| elucidation | component of a cell made of insulating material intended to maintain the spacing between plates of opposite polarity or between the plate pack and the case |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-10 |
| subclassOf | Component |
SpecificCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1e3dc60d_dd6b_47d6_8161_70004fc5ee30
| altLabel | GravimetricCapacity; SpecificChargeCapacity; SpecificElectricChargeCapacity |
| elucidation | quotient of the capacity of a cell or battery [ or electrode or active material ] by its mass. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-19 |
| newareLabel | Capacity Density(mAh/g) |
| restrictions | |
| subclassOf | ISQDerivedQuantity, ElectrochemicalPerformanceQuantity |
| subclasses | DischargingSpecificCapacity, ChargingSpecificCapacity |
SpecificPower#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c762a928_5a74_46fd_9929_4ac2d7a3a8d7
| elucidation | the quotient of the power of an energy-storage device or system and its mass |
| subclassOf | ElectrochemicalPerformanceQuantity |
SpecificSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cf54e7c1_f359_4715_b61d_0350b890d597
| elucidation | total surface of a solid per mass |
| wikidataReference | https://www.wikidata.org/wiki/Q622205 |
| wikipediaReference | https://en.wikipedia.org/wiki/Specific_surface_area |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=485-02-10 |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
Spring#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_872b11e1_8bb3_4a2a_af90_bbaa0055d01e
| elucidation | an elastic device |
| wikidataReference | https://www.wikidata.org/wiki/Q102836 |
| wikipediaReference | https://en.wikipedia.org/wiki/Spring_(device) |
| subclassOf | Component |
| subclasses | BellevilleWasher, WaveSpring |
SquareWaveCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_327eb3e1_f74a_4076_96de_5a2e3f63cb65
| elucidation | component of an electric current that is associated with the presence of an analyate in square-wave voltammetry |
| iupacReference | https://goldbook.iupac.org/terms/view/S05897 |
| subclassOf | ElectricCurrent, ElectrochemicalQuantity |
SquareWavePotentialWaveform#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0e33278b_639f_412d_9abd_64c3790026ef
| elucidation | Periodic potential where the amplitude alternates at a steady frequency between fixed minimum and maximum values, with the same duration at minimum and maximum. |
| subclassOf | ElectricPotentialSignal |
Note
In an ideal square wave, the transitions between minimum and maximum are instantaneous.
SquareWaveVoltammetryWaveform#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_210f3520_1ea3_4c86_aa89_4cd9a3bc5a5a
| elucidation | Square wave potential waveform superimposed to a linear potential ramp or a staircase potential ramp. |
| subclassOf | ElectricPotentialSignal |
StaircaseCurrentRamp#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_626f81db_ae4e_410a_96b8_0582aa2a9434
| elucidation | Successive current steps that form a signal with a staircase waveform. |
| subclassOf | ElectricCurrentSignal |
StaircasePotentialRamp#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d359386f_ae2d_4ad4_9616_464e2111b67d
| elucidation | Successive steps of electric potential that form a staircase waveform. |
| subclassOf | ElectricPotentialSignal |
StandardElectrodePotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7fc10197_41d9_4c1e_a107_928f03eb2d36
| altLabel | StandardPotential |
| elucidation | equilibrium electrode potential of an electrode under standard conditions |
| dbpediaReference | https://dbpedia.org/page/Standard_electrode_potential |
| iupacReference | https://goldbook.iupac.org/terms/view/S05912 |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-02-13 |
| subclassOf | EquilibriumElectrodePotential |
StandardHydrogenElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2a40b878_7d09_49db_91b2_d0ee30192284
| altLabel | SHE |
| elucidation | for solutions in protic solvents, the universal reference electrode for which, under standard conditions, the standard electrode potential (H+ / H2) is zero at all temperatures |
| wikidataReference | https://www.wikidata.org/wiki/Q898559 |
| wikipediaReference | https://en.wikipedia.org/wiki/Standard_hydrogen_electrode |
| iupacReference | https://goldbook.iupac.org/terms/view/S05917 |
| subclassOf | ReferenceElectrode |
Note
Potential of a platinum electrode in a theoretical ideal solution (the current standard for zero potential for all temperatures)
StateOfCharge#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8b2aaa50_bbe1_45da_8778_8898326246a2
| altLabel | SoC |
| elucidation | the ratio of the charged capacity to the rated capacity of an electrochemical device |
| wikipediaReference | https://en.wikipedia.org/wiki/State_of_charge |
| subclassOf | RatioQuantity, ElectrochemicalQuantity |
StateOfHealth#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a7a4614f_2426_46f3_8475_cda4a9fabfce
| elucidation | An indication of the health of an electrochemical device |
| subclassOf | ElectrochemicalQuantity |
StepCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ae108db7_8765_4329_9908_059277dee586
| elucidation | the amount of charge that has passed through an electrochemical device within the time interval of a given testing step |
| subclassOf | Capacity |
StepDuration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_85e39686_9658_4c74_bb91_a935704c174a
| elucidation | the duration between two consecutive steps in a staircase signal |
| subclassOf | Duration, ElectrochemicalControlQuantity |
Note
Typically, the duration is defined between the onset of one step and the onset of the next step.
StepIndex#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d78b696d_9832_4352_a264_28a2ea7d82e4
| altLabel | StepNumber |
| elucidation | the currently running step number in the procedure |
| hiddenLabel | Step |
| maccorLabel | step |
| newareLabel | Step ID |
| arbinLabel | step_index |
| novonixLabel | Step Number |
| subclassOf | PureNumberQuantity, ElectrochemicalQuantity |
StepSignalCurrent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6769536b_5320_4b48_a2d8_ac285ec635a8
| elucidation | the magnitude of the current step in chronopotentiometry and related techniques |
| subclassOf | ElectricCurrent, ElectrochemicalControlQuantity |
StepSignalVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0bf1ed19_2fc9_4e6d_87ec_62015985a9a6
| elucidation | the magnitude of the voltage step in step voltammetry and related techniques |
| restrictions | |
| subclassOf | ElectrochemicalControlQuantity |
StepTime#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_80ca00f8_c891_4493_87a2_7d39b9d1e098
| elucidation | time of a measurement relative to the start of the step (t = 0) |
| hiddenLabel | StepTime/Sec |
| maccorLabel | step (sec) |
| arbinLabel | step_time |
| novonixLabel | Step Time (h) |
| subclassOf | Time, ElectrochemicalQuantity |
StepType#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2d58b059_2ef0_418e_9337_13aa2de4b07f
| altLabel | State |
| elucidation | a description (usually an integer, string, or alpha numeric code) describing the nature of a step in an electrochemical test protocol |
| maccorLabel | state |
| novonixLabel | Step Type |
| subclassOf | ElectrochemicalQuantity |
SternModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_aa5ed981_c11b_4c4f_b1fd_8c432e009484
| elucidation | describes the double layer as divided into two parts separated by the Stern plane, located about one hydrated ion radius from the electrode surface |
| subclassOf | DoubleLayerModel |
Note
proposed by Otto Stern in 1924
StoichiometricCoefficient#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f0667139_6428_4e3d_ac0d_08c1dd7f36ea
| elucidation | the absolute value of the stoichiometric number |
| wikidataReference | https://www.wikidata.org/wiki/Q118455387 |
| wikipediaReference | https://en.wikipedia.org/wiki/Stoichiometry#Stoichiometric_coefficient_and_stoichiometric_number |
| subclassOf | PureNumberQuantity |
| subclasses | StoichiometricCoefficientAtSOC0, StoichiometricCoefficientAtSOC100, MinimumStoichiometricCoefficient, MaximumStoichiometricCoefficient |
StoichiometricCoefficientAtSOC0#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f22bd1ec_faca_4335_92a5_a1687154c622
| elucidation | the stoichiometric coefficient for a reactive entity in a chemical substance that corresponds to a cell-level state-of-charge of 0% |
| subclassOf | StoichiometricCoefficient |
| subclasses | PositiveElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC0, NegativeElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC0 |
StoichiometricCoefficientAtSOC100#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_38ab058e_3912_48c2_a7eb_76d25d000820
| elucidation | the stoichiometric coefficient for a reactive entity in a chemical substance that corresponds to a cell-level state-of-charge of 100% |
| subclassOf | StoichiometricCoefficient |
| subclasses | NegativeElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC100, PositiveElectrodeActiveMaterialGuestStoichiometricCoefficientAtSOC100 |
StoichiometricEquation#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1e72986e_e19f_4c24_8663_cadd4318bd72
| altLabel | ChemicalEquation; ChemicalReactionEquation |
| elucidation | the symbolic representation of a chemical reaction in the form of symbols and formulae, wherein the reactant entities are given on the left-hand side and the product entities on the right-hand side |
| wikidataReference | https://www.wikidata.org/wiki/Q182527 |
| wikipediaReference | https://en.wikipedia.org/wiki/Chemical_equation |
| dbpediaReference | https://dbpedia.org/page/Chemical_equation |
| iupacReference | https://doi.org/10.1351/goldbook.C01034 |
| subclassOf | Mathematical, ChemicalSymbolicConstruct |
StorageTest#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c91164b8_5e56_4c94_bad1_d7ada576b0e7
| altLabel | SelfDischargeTest |
| elucidation | test carried out to measure the loss of capacity, open-circuit voltage, short-circuit current or other quantities after storage under specified conditions |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-45 |
| restrictions |
|
| subclassOf | Electrochemical |
StoredEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_46376e5d_9627_4514_9881_9e62083625c3
| altLabel | BatteryEnergy; EnergyStorageCapability |
| elucidation | amount of energy that is passed to or from an energy storage device under specified conditions |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-21 |
| hiddenLabel | Energy/mWh; Watt-hr; mWatt-hr |
| maccorLabel | watt-hr |
| newareLabel | Energy(mWh) |
| indigoLabel | cell_energy_j |
| novonixLabel | Energy (Wh) |
| subclassOf | Energy, ElectrochemicalPerformanceQuantity |
| subclasses | DischargingEnergy, RatedEnergy, ChargingEnergy, TotalEnergy |
StrippingChronopotentiometry#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bfbefff0_4df5_47c2_9943_5f42cf268e9e
| elucidation | an electroanalytical technique that involves applying a constant current to a working electrode to strip previously deposited analyte from the electrode surface while measuring the potential change over time |
| subclassOf | CurrentControlledProcess |
StrontiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_46ac0fd3_2b8e_40aa_bf5d_19cf1dd39052
| elucidation | an electrode in which the primary active material consists of strontium or strontium compounds |
| subclassOf | ActiveElectrode |
Substrate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_51c9716c_75c8_40d1_a782_c6810bda1433
| elucidation | the underlying material or surface onto which a coating, layer, or treatment is applied, serving as the base that supports and interacts with the added material. |
| wikipediaReference | https://en.wikipedia.org/wiki/Substrate_(materials_science) |
| iupacReference | https://goldbook.iupac.org/terms/view/S06084 |
| subclassOf | Component |
SulfurBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_88d2d4bc_4244_4419_a260_ad099a62d580
| elucidation | an electrode in which the primary active material consists of sulfur or sulfur compounds |
| subclassOf | ActiveElectrode |
| subclasses | ThionylChlorideElectrode |
SulfuricAcidSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_334b3acd_e35d_4d5a_b8b5_6d13cbebbc57
| elucidation | a solution of sulfuric acid (H2SO4) dissolved in water (H2O) |
| subclassOf | AcidicElectrolyte |
Supercapacitor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_dc6abae7_39b0_4d22_b52e_14d088a4bd5f
| altLabel | Supercap |
| elucidation | type of capacitor characterized by high capacitance, high specific and volumetric energy density, and low voltage limits |
| wikidataReference | https://www.wikidata.org/wiki/Q754523 |
| wikipediaReference | https://en.wikipedia.org/wiki/Supercapacitor |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-03 |
| subclassOf | ElectrochemicalDevice |
| subclasses | ElectrochemicalDoubleLayerCapacitor, ElectrochemicalPseudocapacitor |
SupportingElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1fc5642c_b7b2_43bf_ad20_f96001db8800
| altLabel | BaseElectrolyte; IndifferentElectrolyte; InertElectrolyte |
| elucidation | electrolyte solution, the ions of which are electroinactive in the range of applied potential being studied, and whose ionic strength (and, therefore, contribution to the overall conductivity) is usually much greater than the concentration of an electroactive substance to be dissolved in it. |
| wikipediaReference | https://en.wikipedia.org/wiki/Supporting_electrolyte |
| iupacReference | https://goldbook.iupac.org/terms/view/S06149 |
| subclassOf | ElectrolyteSolution |
SurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_05cf26ef_782a_4f66_a196_7004dd973f8e
| elucidation | a measure of the total area that the surface of a solid object occupies |
| wikidataReference | https://www.wikidata.org/wiki/Q1379273 |
| wikipediaReference | https://en.wikipedia.org/wiki/Surface_area |
| dbpediaReference | https://dbpedia.org/page/Surface_area |
| subclassOf | Area, ElectrochemicalQuantity |
| subclasses | ElectrodeSurfaceArea, DeviceSurfaceArea |
SurfaceAreaPerVolume#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7f381c19_cf07_47a8_ab10_0b14d46901e8
| elucidation | the quotient of the surface area of some object and its total volume |
| subclassOf | ISQDerivedQuantity |
SurfaceOverpotential#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_60741c58_a10d_4aa6_bb68_0066a6ff8e30
| altLabel | SurfacePolarization |
| elucidation | The potential of a working electrode relative to a reference electrode of the same kinds placed in the solution adjacent to the surface of the working electrode (just outside the double layer). |
| restrictions | |
| subclassOf | Overpotential |
SwagelokCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cd1b7943_42ce_46bd_8588_1c3161268270
| elucidation | a container for an electrochemical cell manufactured by the company Swagelok |
| subclassOf | Case |
TabOffset#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9171ef9b_65b6_4536_9a1b_cb2b38961311
| elucidation | the perpendicular distance between the nearest pair of parallel edges belonging to the tab base and the current collector |
| illustration |  |
| subclassOf | Distance |
TappedDensity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5ce6a328_713c_4383_ad63_26c902c30e34
| altLabel | TapDensity |
| elucidation | the density of a powdered or granular material when it is subjected to tapping or mechanical vibration |
| subclassOf | Density |
TemperatureCoefficientOfTheCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8f3ab19f_ce22_424e_a9bf_d5cedb815374
| elucidation | quotient of the change in capacity of a cell by the corresponding change in temperature. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-18 |
| subclassOf | ElectrochemicalQuantity |
TemperatureCoefficientOfTheOpenCircuitVoltage#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3bb5ae23_59fa_4bc7_9495_803eb6719f28
| elucidation | quotient of change in open-circuit voltage of a cell or battery by the corresponding change in temperature. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-33 |
| subclassOf | ElectrochemicalQuantity |
| subclasses | NegativeElectrodeEntropicChangeCoefficient, PositiveElectrodeEntropicChangeCoefficient |
TemperatureLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_82fce40c_434d_4640_a3d2_e26379b6acae
| elucidation | limit on the temperature of a system |
| subclassOf | CelsiusTemperature, LimitQuantity |
| subclasses | MaximumTemperature, MiniumumTemperature |
Terminal#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3f70e5c5_67f0_483e_998b_b83b7739c4bc
| elucidation | conductive part of a device, electric circuit or electric network, provided for connecting that device, electric circuit or electric network to one or more external conductors |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-22 |
| subclassOf | Component |
| subclasses | NegativeTerminal, PositiveTerminal |
TerminalProtector#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_60bc27f0_8290_4113_8c3b_011433a733c7
| altLabel | TerminalCover |
| elucidation | cover of insulating material used to avoid electric contact with the cell or battery terminal |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-23 |
| subclassOf | Component |
TerminationQuantity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_44a67eb4_52e8_433c_aef7_a928c2b9906c
| elucidation | a quantity that is used to express the termination criteria for an electrochemical process |
| subclassOf | ElectrochemicalControlQuantity |
TestTime#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_27b3799c_250c_4332_8b71_7992c4a7bb05
| altLabel | RunTime |
| elucidation | time of a measurement relative to the start of the test (t = 0) |
| hiddenLabel | TestTime/Sec; time/s |
| maccorLabel | test (sec) |
| batteryArchiveLabel | Test_Time (s) |
| newareLabel | Time(h:min:s.ms) |
| indigoLabel | time_s |
| arbinLabel | test_time |
| novonixLabel | Run Time (h) |
| subclassOf | Time, ElectrochemicalQuantity |
TheoreticalCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_372c89d0_adab_4585_9662_33c912acef23
| elucidation | the amount of electric charge that can be stored in a material, component, or device, under infinitely low current, and derived from first principles |
| typicalUnit | https://w3id.org/emmo#AmpereHour; https://w3id.org/emmo#MilliAmpereHour |
| subclassOf | RatedCapacity |
Caution
The value of the practically achieve able capacity is often much lower than the theoretical value.
ThermalRunaway#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_04f28ce3_251d_429e_aa85_ab3eb45bbcd2
| elucidation | unstable condition arising during constant voltage charge in which the rate of heat dissipation capability, causing a continuous temperature increase with resulting further charge current increase, which can lead to the destruction of the battery |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-54 |
| subclassOf | ElectrochemicalPhenomenon |
Note
in lithium batteries thermal runaway may cause melting of lithium
ThermogalvanicCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_113e0469_8ae0_407f_892d_4b988f8d8a08
| altLabel | Thermocell |
| elucidation | electrochemical cell that has two half-cells separated by a wall permeable to ions, both containing the same electrolyte differing only in their temperatures. |
| wikidataReference | https://www.wikidata.org/wiki/Q25304247 |
| wikipediaReference | https://en.wikipedia.org/wiki/Thermogalvanic_cell |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=114-03-09 |
| subclassOf | ElectrochemicalDevice |
ThionylChlorideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4977521c_0438_4659_bc81_1c77fae836bb
| elucidation | an electrode in which the primary active material consists of thionyl chloride |
| subclassOf | SulfurBasedElectrode |
Note
the combination with lithium metal and non-aqueous in-organic electrolyte is designated using IEC electrochemical system letter code E
ThreeElectrodeElectrochemicalCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b9bece97_a511_4cb9_88a2_b5bd5c5e5d74
| elucidation | electrochemical cell with a working electrode, reference electrode, and auxiliary electrode |
| subclassOf | ElectrochemicalCell |
Note
Electric current flows between the working and auxiliary electrodes. Electrode potential may be measured between the working and reference electrodes.
Note
The working electrode is the site of interest where the electrochemical process occurs, the reference electrode provides a stable potential, and the auxiliary (counter) electrode completes the circuit.
Tip
A potentiostat can be used to maintain a potential difference between the working and reference electrodes.
TimeData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_58ad1d22_3803_4c95_a137_207cfebe242a
| elucidation | time data, usually resulting from an electrochemical measurement process |
| restrictions |
|
| subclassOf | Vector, RawData |
TimeMeasurement#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7fdf65c1_b86a_4cbe_8c06_56321b425d4e
| elucidation | measurement of time |
| restrictions | |
| subclassOf | Measurement |
TimeMeasurementResult#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_496c8f31_9732_42c1_8eae_dd73979c06eb
| elucidation | the reseult of a time measurement |
| subclassOf | MeasurementResult |
TimeSeriesDataSet#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7fa74f69_047f_4c86_a02c_e7805d6f5b77
| elucidation | a series of data points indexed in time order |
| wikidataReference | https://www.wikidata.org/wiki/Q186588 |
| wikipediaReference | https://en.wikipedia.org/wiki/Time_series |
| restrictions |
|
| subclassOf | Dataset, Matrix |
| subclasses | TimeSeriesElectricalDataSet |
TimeSeriesElectricalDataSet#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_31e8052d_bede_43c6_8b41_d51bb24c9489
| elucidation | a time series of electrical quantities |
| restrictions | |
| subclassOf | TimeSeriesDataSet |
| subclasses | GalvanostaticIntermittentTitrationTechniqueData, PseudoOpenCircuitVoltageData, PulseResponseData |
TinBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a10ede13_c895_4f56_a728_b1aab512b31b
| elucidation | an electrode in which the primary active material consists of tin or tin compounds |
| subclassOf | ActiveElectrode |
TitaniumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9c557caa_61e2_4fa9_a517_4bad01a68122
| elucidation | an electrode in which the primary active material consists of titanium or titanium compounds |
| subclassOf | ActiveElectrode |
| subclasses | SodiumMetatitanateElectrode, LithiumTitanateElectrode, SodiumTitaniumOxideElectrode, SodiumTitaniumPhosphateElectrode, TitaniumDioxideElectrode |
Note
often represented in cell designations by the letter T
Note
this class is intended to enable designations based on IEC recommendations
TitaniumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d90221a0_0da7_4876_9cac_0e943e445f6f
| elucidation | an electrode in which the primary active material consists of titanium or titanium compounds |
| subclassOf | ActiveElectrode |
TitaniumDioxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_76fe8fb2_868e_48eb_95ca_fc6acd6f5fc9
| altLabel | TiO2Electrode |
| elucidation | electrode in which the active material is titanium dioxide |
| restrictions | |
| subclassOf | TitaniumBasedElectrode, MetalOxideElectrode |
TotalDuration#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4a30ff40_3ffa_4a08_9134_36dbe718bee1
| elucidation | the total duration of a test |
| subclassOf | Duration |
TotalEnergy#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0f986d97_445f_4075_a5ce_ddde598a47a9
| elucidation | the total amount of energy that has passed through an electrochemical device |
| subclassOf | StoredEnergy |
TotalMassLoading#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8541f8b7_c55e_452d_b3f8_db29dcb2a61d
| elucidation | the mass of all material (active and inactive) per unit area |
| subclassOf | MassLoading |
TotalNumberOfCycles#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6d981c04_3ace_4f1b_b0f8_770776cceb6f
| elucidation | the total number of cycles carried out on a specific device under a given set of conditions |
| subclassOf | PureNumberQuantity, ElectrochemicalQuantity |
TransitionTime#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ae9882de_d6a2_4525_a84f_80947c10f1cb
| elucidation | in chronopotentiometry and related techniques, the duration between the application of current and when the concentration of an electroactive substance at the electrode-solution interface becomes indistinguishable from zero |
| iupacReference | https://doi.org/10.1351/goldbook.T06472 |
| subclassOf | ElectrochemicalQuantity |
TrasattiBuzzancaModel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f57a7dac_2ec2_4d51_b697_01a844c4467f
| elucidation | considers the effects of specific adsorbed ions in the double layer |
| subclassOf | DoubleLayerModel |
Note
proposed by Sergio Trasatti and Giovanni Buzzanca in 1971
TriangularCurrentWaveform#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_09b9a369_2228_44bd_9b63_8abecfec8650
| elucidation | Linear ramp of electric current followed by a reversal to the initial current (typically I=0), both at the same current change rate. |
| subclassOf | ElectricCurrentSignal |
TriangularPotentialWaveform#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f9af8440_3629_4558_a944_9dfaf3dfd7ec
| elucidation | Linear ramp of electric potential, followed by a reversal to the initial potential. Both forward and reversed signal ramps are applied at the same scan rate. |
| subclassOf | ElectricPotentialSignal |
TubularPlate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6b1f594f_2c0a_46a0_8805_d6ab08baa75e
| elucidation | positive plate (electrode) which is either composed of an assembly of porous tube of perforated metal or tissue either a gauntlet with or without a central current collector spine, the active material being placed within the tubes |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-07 |
| subclassOf | PositiveElectrode |
TungstenBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c5fd7b61_40f1_4225_a173_5caa3c5f4773
| elucidation | an electrode in which the primary active material consists of tungsten or tungsten compounds |
| subclassOf | ActiveElectrode |
TungstenOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3fdc81d5_eef3_4408_a6e4_3c51c5b1c8dc
| altLabel | WO3Electrode |
| elucidation | electrode in which the active material is tungsten oxide |
| subclassOf | MetalOxideElectrode |
TwoStepCharging#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_aac51107_dbe5_4e63_b08a_9d6cf88f4b69
| elucidation | charging method applied to a secondary battery using two levels of charge rate with feedback control to initiate the changeover from a high to a low charge rate |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-48 |
| subclassOf | SerialWorkflow, Charging |
| subclasses | ConstantCurrentConstantVoltageCharging |
UpperCurrentDensityLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2a16f139_ab9c_4034_bd21_eabd2e1202e8
| elucidation | the upper bounding limit placed on the current density used in the definition of an electrochemical testing protocol |
| subclassOf | CurrentDensityLimit |
UpperCurrentLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3dac5115_6337_40a6_a2ab_317087fe3b45
| elucidation | the upper bounding limit on the current used in the definition of an electrochemical testing protocol |
| subclassOf | CurrentLimit |
UpperFrequencyLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_666f0b69_9c74_49a3_80b3_96a188332462
| elucidation | the higher end of the interval of frequencies tested in impedimetry and related techniques |
| subclassOf | Frequency, ElectrochemicalControlQuantity |
UpperVoltageLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6dcd5baf_58cd_43f5_a692_51508e036c88
| altLabel | EndOfChargeVoltage; UpperCutoffVoltage |
| elucidation | maximum voltage limit at which an applied excitation is altered or terminated |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-55 |
| subclassOf | VoltageLimit |
Note
term used in the context of primary and secondary cells and batteries
VMP300#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9e9a7e39_9247_4323_b4f1_0aa314a89a34
| altLabel | VMP-300 |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
VMP3e#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_da379716_1f94_4c70_a6f7_bcb55f8050ad
| altLabel | VMP-3e |
| elucidation | a potentiostat / galvanostat manufactured by BioLogic |
| subclassOf | Galvanostat, Potentiostat |
VSP#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e5ac23cb_f563_4ae5_8d2b_bf54853fc4d8
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
VSP300#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fec9e6b2_5319_4931_a2d6_bd27b8d462a5
| altLabel | VSP-300 |
| elucidation | a potentiostat manufactured by BioLogic |
| subclassOf | Potentiostat |
VSP3e#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_77524199_0925_4bd0_82d3_bfef58fa1bc1
| altLabel | VSP-3e |
| elucidation | a potentiostat / galvanostat manufactured by BioLogic |
| subclassOf | schema.org.Product, Galvanostat, Potentiostat |
Valve#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a2f4dca3_829f_412b_9025_bd7581aad82a
| elucidation | component of a cell which permits the flow of gas in one direction only |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-02-12 |
| subclassOf | Component |
Note
a valve has a characteristic venting or opening and closing pressure
VanadiumBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ee59786_b090_444d_a46d_505797d07ca4
| elucidation | an electrode in which the primary active material consists of vanadium or vanadium compounds |
| subclassOf | ActiveElectrode |
| subclasses | LithiumVanadiumOxideElectrode |
Note
often represented in IEC cell designations by the letter V
Note
this class is intended to enable designations based on IEC recommendations
VentCap#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cc1f854a_ba6c_436e_bd18_c8b9159bbf52
| elucidation | component fitted into the filling hole of a cell with a provision of allowing the venting of electrolysis gas from the cell¨ |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-23 |
| subclassOf | Component |
VentedCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ec1dce8b_bb46_41a9_b532_6bed381aa557
| elucidation | secondary cell having a cover provided with an opening through which products of electrolysis and evaporation are allowed to escape freely from the cell to the atmosphere |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-05-14 |
| subclassOf | ElectrochemicalDevice |
VoltageChangeLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_09e64707_a17d_4405_84cc_ee9d91ed32ef
| altLabel | dV/dt |
| elucidation | a control limit placed on the rate of change of the cell voltage, dV/dt. |
| restrictions | |
| subclassOf | ElectrochemicalControlQuantity, VoltageChangeRate |
VoltageChangeRate#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f6f45881_9bf1_4cf5_988b_de79dab718ef
| altLabel | dV/dt |
| elucidation | the first order time rate of change of voltage |
| arbinLabel | dv/dt |
| novonixLabel | dVdt (I/h) |
| restrictions | |
| subclassOf | ElectrochemicalQuantity |
| subclasses | VoltageChangeLimit |
VoltageControlledProcess#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_46957d35_0f8b_4d92_acb3_aded6ce774a1
| elucidation | a process in which the voltage is controlled |
| subclassOf | Process |
| subclasses | VoltageHold, PotentiostaticCycling |
VoltageData#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4d09ddc4_7e71_4712_afab_b33d8df38983
| elucidation | voltage data, usually resulting from an electrochemical measurement process |
| restrictions | |
| subclassOf | Vector, RawData |
VoltageHold#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_f07be701_9d6a_415b_ac6d_63202297a7a1
| altLabel | PotentiostaticProcess |
| elucidation | a process in which the voltage is kept constant |
| restrictions | |
| subclassOf | VoltageControlledProcess |
| subclasses | ConstantVoltageDischarging, FloatCharging, ConstantVoltageCharging |
VoltageLimit#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5ebeea80_af22_456b_9087_78c2a8465c58
| elucidation | limit on the voltage of an electrical system |
| subclassOf | Voltage, LimitQuantity |
| subclasses | UpperVoltageLimit, electrochemistry.LowerVoltageLimit |
VoltageMeasurement#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_825a749f_dd07_46fb_947c_db286440911e
| altLabel | ElectrochemicalCellVoltageMeasurement |
| elucidation | measurement of the electric potential between the terminals of an electrochemical cell |
| restrictions | |
| subclassOf | Measurement |
VoltageMeasurementResult#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a76072fe_ca6e_48ad_8b4a_ab98a2bc9abb
| elucidation | the result of a voltage measurement |
| subclassOf | MeasurementResult |
Voltammogram#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_bbae1ef4_0a95_4e7d_9932_1bf939129eef
| elucidation | current-potential curve in a voltammetry experiment |
| wikipediaReference | https://en.wikipedia.org/wiki/Voltammetry#Voltammograms |
| subclassOf | CurrentPotentialPlot |
Voltmeter#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1355816f_a2b5_4800_8001_fc888f5d6b1b
| elucidation | instrument intended to measure the value of a voltage. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=313-01-03 |
| subclassOf | MeasuringInstrument |
VolumePercent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_db37b358_b2f0_4e06_b6ae_8c56c8fbb6ba
| elucidation | the ratio of a constituent volume to the total volume |
| subclassOf | PercentQuantity |
VolumetricCapacity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e6b63190_acac_4e78_9cba_fb2b10bbe910
| altLabel | VolumetricChargeCapacity; VolumetricElectricChargeCapacity |
| elucidation | quotient of the capacity of a cell or battery by its volume. |
| IEVReference | https://www.electropedia.org/iev/iev.nsf/display?openform&ievref=482-03-17 |
| subclassOf | ElectrochemicalQuantity |
VolumetricSurfaceArea#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a5571263_f153_448f_84a3_cd18092cf8fa
| altLabel | SurfaceAreaToVolumeRatio |
| elucidation | ratio of the surface area of a solid object to the volume of the object |
| wikidataReference | https://www.wikidata.org/wiki/Q766786 |
| wikipediaReference | https://en.wikipedia.org/wiki/Surface-area-to-volume_ratio |
| subclassOf | ElectrochemicalQuantity |
| subclasses | NegativeElectrodeActiveMaterialVolumetricSurfaceArea, PositiveElectrodeActiveMaterialVolumetricSurfaceArea |
Water#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a7b05629_80d7_402b_be1a_69933d24c4cb
| altLabel | H2O |
| elucidation | a chemical compound consisting of two hydrogen atoms bonded to one oxygen atom |
| wikidataReference | https://www.wikidata.org/wiki/Q283 |
| pubChemReference | https://pubchem.ncbi.nlm.nih.gov/compound/Water#section=Related-CAS |
| wikipediaReference | https://en.wikipedia.org/wiki/Water |
| subclassOf | AmphotericCompound |
WattPerWattHour#
IRI: https://w3id.org/emmo/domain/electrochemistry#WattPerWattHour
| elucidation | the unit for P-Rate in electrochemistry and related domains |
| subclassOf | PRateUnit |
WaveSpring#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6385e07f_f40d_46b2_b998_c439950d75cb
| elucidation | a spring made of pre-hardened flat wire, formed into a wavy washer shape |
| wikidataReference | https://www.wikidata.org/wiki/Q11288626 |
| wikipediaReference | https://en.wikipedia.org/wiki/Wave_spring |
| subclassOf | Spring |
WeightPercent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_2c0e66c8_d58d_44b2_b0ce_ba55194bd505
| elucidation | the percentage quantity relating the weight of a constituent to the total weight |
| subclassOf | PercentQuantity |
WorkingElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fb988878_ee54_4350_9ee9_228c00c3ad35
| elucidation | electrode at which one or more electroactive substances undergo reaction in the solution being investigated |
| wikidataReference | https://www.wikidata.org/wiki/Q477099 |
| wikipediaReference | https://en.wikipedia.org/wiki/Working_electrode |
| iupacReference | https://goldbook.iupac.org/terms/view/W06686 |
| subclassOf | Electrode |
| subclasses | AnnularWorkingElectrode |
Note
If processes of interest occur both at the anode and the cathode of a cell (as in differential amperometry or controlled-current potentiometric titration with two indicator electrodes), the cell should be said to comprise two indicator or working electrodes.
Note
Te potential of a working electrode is controlled with respect to the reference electrode.
Note
This term is typically used in voltammetry, polarography, amperometry, and coulometry.
WorkingPotentialRange#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c39b2498_783e_48e1_9814_6164bd99823c
| elucidation | range of electrode potentials of a given working electrode in a given electrolyte, where the electric current from reactions of the electrode or electrolyte is negligible compared with the current from reactions of the system under investigation |
| subclassOf | ElectricPotential, ElectrochemicalQuantity |
WovenMesh#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3a69a00e_235a_4948_b369_8ac20116c85e
| elucidation | a fabric-like structure created by interweaving wires or fibres in an over-under pattern, commonly used for filtration, screening, and reinforcement due to its high strength and durability |
| subclassOf | ManufacturedMaterial |
ZincBasedElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_d0a26dc2_fde9_4a11_ac26_7c18499d28a5
| elucidation | an electrode in which the primary active material consists of zinc or zinc compounds |
| subclassOf | ActiveElectrode |
| subclasses | ZincElectrode |
ZincChlorideSolution#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7393f12f_e3b9_42d6_bffb_e5613f53108f
| altLabel | AqueousZincChlorideSolution |
| elucidation | a solution of zinc chloride (ZnCl2) dissolved in water (H2O) |
| subclassOf | NearNeutralElectrolyte |
ZincElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_424bf750_7df5_49b5_ba73_ba74397a166b
| elucidation | an electrode in which the main active material is zinc metal |
| restrictions |
|
| subclassOf | MetalElectrode, ZincBasedElectrode |
Note
the combination with manganese dioxide and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code L
Note
the combination with nickel oxide hydroxide and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code Z
Note
the combination with oxygen and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code P
Note
the combination with oxygen and an ammonium chloride zinc chloride electrolyte is designated using IEC electrochemical system letter code A
Note
the combination with silver oxide and an alkali metal hydoxide electrolyte is designated using IEC electrochemical system letter code S
ZincInsertionElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_245c9442_ca1d_4070_a624_182b92d30b10
| elucidation | an insertion electrode in which the guest molecule is zinc |
| subclassOf | InsertionElectrode |
ZincOxideElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_82c70935_0aea_454b_92bf_17fb0b488012
| altLabel | ZnOElectrode |
| elucidation | electrode in which the active material is zinc oxide |
| restrictions | |
| subclassOf | MetalOxideElectrode |
arbinLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a07dd85b_d0df_4d5d_8b0c_14d20ea3fe45
| elucidation | the label of a term used in resources from Arbin |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
arbinLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_a07dd85b_d0df_4d5d_8b0c_14d20ea3fe45
| elucidation | the label of a term used in resources from Arbin |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
batteryArchiveLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1b717a20_17e3_4480_8c60_c4ae94058f05
| elucidation | the label of a term used in resources from Battery Archive |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
batteryArchiveLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1b717a20_17e3_4480_8c60_c4ae94058f05
| elucidation | the label of a term used in resources from Battery Archive |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
battmoKey#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e5e86474_8623_48ea_a1cf_502bdb10aa14
| subclassOf | owl.AnnotationProperty, rdf-schema.label |
battmoKey#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e5e86474_8623_48ea_a1cf_502bdb10aa14
| subclassOf | owl.AnnotationProperty, rdf-schema.label |
biologicLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_69022178_77b8_4669_97e2_351d45356e5a
| elucidation | the label of a term used in resources from Biologic |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
biologicLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_69022178_77b8_4669_97e2_351d45356e5a
| elucidation | the label of a term used in resources from Biologic |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
bpxKey#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a5b99ee_995b_4899_a79b_925a4086da37
| subclassOf | owl.AnnotationProperty, rdf-schema.label |
bpxKey#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0a5b99ee_995b_4899_a79b_925a4086da37
| subclassOf | owl.AnnotationProperty, rdf-schema.label |
bvco#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c1ac455d_964c_4435_a9f0_d7339c44f4bb
| subclassOf | owl.AnnotationProperty, rdf-schema.seeAlso |
bvco#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c1ac455d_964c_4435_a9f0_d7339c44f4bb
| subclassOf | owl.AnnotationProperty, rdf-schema.seeAlso |
cidemodKey#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1b718841_5d72_4071_bb71_fc4a754f5e30
| subclassOf | owl.AnnotationProperty, rdf-schema.label |
cidemodKey#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1b718841_5d72_4071_bb71_fc4a754f5e30
| subclassOf | owl.AnnotationProperty, rdf-schema.label |
hasANSICode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eac15067_9594_4eab_a440_b537b0fd2075
| subclassOf | owl.DatatypeProperty |
hasANSICode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_eac15067_9594_4eab_a440_b537b0fd2075
| subclassOf | owl.DatatypeProperty |
hasActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_860aa941_5ff9_4452_8a16_7856fad07bee
| elucidation | relates an electrode or other functional component to the material that participates directly in the primary electrochemical reaction |
| subclassOf | owl.ObjectProperty, hasComponent |
hasActiveMaterial#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_860aa941_5ff9_4452_8a16_7856fad07bee
| elucidation | relates an electrode or other functional component to the material that participates directly in the primary electrochemical reaction |
| subclassOf | owl.ObjectProperty, hasComponent |
hasAdditive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7df82c48_b599_4b02_bef0_9facc9c39410
| elucidation | relates a component or mixture to an additive that is included in minor quantity to modify physical, chemical, or electrochemical properties |
| subclassOf | owl.ObjectProperty, hasComponent |
| subclasses | hasConductiveAdditive |
hasAdditive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7df82c48_b599_4b02_bef0_9facc9c39410
| elucidation | relates a component or mixture to an additive that is included in minor quantity to modify physical, chemical, or electrochemical properties |
| subclassOf | owl.ObjectProperty, hasComponent |
| subclasses | hasConductiveAdditive |
hasAnolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ac306fd_e98b_4af9_8cd6_5ed34f7f7d8a
| elucidation | relates a system to the portion of the electrolyte that is in contact with the anode |
| subclassOf | owl.ObjectProperty, hasElectrolyte |
hasAnolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_0ac306fd_e98b_4af9_8cd6_5ed34f7f7d8a
| elucidation | relates a system to the portion of the electrolyte that is in contact with the anode |
| subclassOf | owl.ObjectProperty, hasElectrolyte |
hasBinder#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_056a5fab_3d99_46bd_8eb1_6e89a368e1a7
| elucidation | relates a composite material or coating to a binder that provides mechanical cohesion and adhesion between its constituents |
| subclassOf | owl.ObjectProperty, hasComponent |
hasBinder#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_056a5fab_3d99_46bd_8eb1_6e89a368e1a7
| elucidation | relates a composite material or coating to a binder that provides mechanical cohesion and adhesion between its constituents |
| subclassOf | owl.ObjectProperty, hasComponent |
hasCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3dcfe33d_6825_43c0_a798_68e871a68d39
| elucidation | relates a device or assembly to the outer enclosure or housing that provides mechanical protection and containment |
| subclassOf | owl.ObjectProperty, hasComponent |
hasCase#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3dcfe33d_6825_43c0_a798_68e871a68d39
| elucidation | relates a device or assembly to the outer enclosure or housing that provides mechanical protection and containment |
| subclassOf | owl.ObjectProperty, hasComponent |
hasCatholyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b62a6b15_236a_48bf_a323_ccbfc295f934
| elucidation | relates a system to the portion of the electrolyte that is in contact with the cathode |
| subclassOf | owl.ObjectProperty, hasElectrolyte |
hasCatholyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_b62a6b15_236a_48bf_a323_ccbfc295f934
| elucidation | relates a system to the portion of the electrolyte that is in contact with the cathode |
| subclassOf | owl.ObjectProperty, hasElectrolyte |
hasCoating#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4df9926d_d4f2_4955_93f3_a03c5edc5383
| elucidation | relates a component to a coating that covers its surface to provide protective, functional, or structural properties |
| subclassOf | owl.ObjectProperty, hasConstituent |
hasCoating#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_4df9926d_d4f2_4955_93f3_a03c5edc5383
| elucidation | relates a component to a coating that covers its surface to provide protective, functional, or structural properties |
| subclassOf | owl.ObjectProperty, hasConstituent |
hasConductiveAdditive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c830c469_60c3_4380_8382_4df13a32a1e7
| elucidation | relates a component or mixture to an additive that is intendend to increase the conductivity of the whole. |
| subclassOf | owl.ObjectProperty, hasAdditive |
hasConductiveAdditive#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c830c469_60c3_4380_8382_4df13a32a1e7
| elucidation | relates a component or mixture to an additive that is intendend to increase the conductivity of the whole. |
| subclassOf | owl.ObjectProperty, hasAdditive |
hasControlParameter#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e55f2798_55c8_4fc5_9abb_2f8ac101f3b8
| elucidation | a property that links a task to a parameter that is controlled during the task |
| subclassOf | owl.ObjectProperty, hasInput |
hasControlParameter#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e55f2798_55c8_4fc5_9abb_2f8ac101f3b8
| elucidation | a property that links a task to a parameter that is controlled during the task |
| subclassOf | owl.ObjectProperty, hasInput |
hasCounterElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e08d43cf_93e4_49c6_bcb6_472be52ae7fc
| elucidation | relates an electrochemical cell or setup to the electrode that completes the electrical circuit and serves as a reference or counter to the working electrode |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasCounterElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e08d43cf_93e4_49c6_bcb6_472be52ae7fc
| elucidation | relates an electrochemical cell or setup to the electrode that completes the electrical circuit and serves as a reference or counter to the working electrode |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasCurrentCollector#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cc8c2c5d_cf3d_444d_a7e8_44ec4c06a88e
| subclassOf | owl.ObjectProperty, hasComponent |
hasCurrentCollector#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_cc8c2c5d_cf3d_444d_a7e8_44ec4c06a88e
| subclassOf | owl.ObjectProperty, hasComponent |
hasElectrochemicalCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1784d824_15bd_4eff_9e40_61e23be6f979
| subclassOf | owl.ObjectProperty, hasComponent |
hasElectrochemicalCell#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1784d824_15bd_4eff_9e40_61e23be6f979
| subclassOf | owl.ObjectProperty, hasComponent |
hasElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_578c41e9_ee01_4840_9c8c_04ab6e4e6241
| elucidation | relates an electrochemical cell or subsystem to an electrode that participates in electron transfer reactions |
| subclassOf | owl.ObjectProperty, hasComponent |
| subclasses | hasWorkingElectrode, hasCounterElectrode, hasPositiveElectrode, hasReferenceElectrode, hasNegativeElectrode |
hasElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_578c41e9_ee01_4840_9c8c_04ab6e4e6241
| elucidation | relates an electrochemical cell or subsystem to an electrode that participates in electron transfer reactions |
| subclassOf | owl.ObjectProperty, hasComponent |
| subclasses | hasWorkingElectrode, hasCounterElectrode, hasPositiveElectrode, hasReferenceElectrode, hasNegativeElectrode |
hasElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3bd08946_4e81_455d_9fca_dc7a5ead9315
| elucidation | relates an electrochemical cell or system to the electrolyte that enables ionic conduction between electrodes |
| subclassOf | owl.ObjectProperty, hasComponent |
| subclasses | hasAnolyte, hasCatholyte |
hasElectrolyte#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_3bd08946_4e81_455d_9fca_dc7a5ead9315
| elucidation | relates an electrochemical cell or system to the electrolyte that enables ionic conduction between electrodes |
| subclassOf | owl.ObjectProperty, hasComponent |
| subclasses | hasAnolyte, hasCatholyte |
hasFormFactor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c17b514b_57f2_4f72_9b95_c2950dd2aa7f
| subclassOf | owl.ObjectProperty, hasProperty |
hasFormFactor#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c17b514b_57f2_4f72_9b95_c2950dd2aa7f
| subclassOf | owl.ObjectProperty, hasProperty |
hasIECCode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1f2d401c_eede_4178_9ff5_e5392bc2cb92
| subclassOf | owl.DatatypeProperty |
hasIECCode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_1f2d401c_eede_4178_9ff5_e5392bc2cb92
| subclassOf | owl.DatatypeProperty |
hasInsertedEntity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fd1fae29_a182_4692_98b8_919764b07b00
| subclassOf | owl.ObjectProperty, hasConstituent |
hasInsertedEntity#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_fd1fae29_a182_4692_98b8_919764b07b00
| subclassOf | owl.ObjectProperty, hasConstituent |
hasLayer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6164a2b0_7fd7_4059_8567_3dcce90b1dec
| elucidation | relates a system or component to a layer that forms part of its structural composition, typically distinguished by its material or functional characteristics |
| subclassOf | owl.ObjectProperty, hasComponent |
hasLayer#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_6164a2b0_7fd7_4059_8567_3dcce90b1dec
| elucidation | relates a system or component to a layer that forms part of its structural composition, typically distinguished by its material or functional characteristics |
| subclassOf | owl.ObjectProperty, hasComponent |
hasNegativeElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5d299271_3f68_494f_ab96_3db9acdd3138
| elucidation | relates an electrochemical cell to the electrode at which oxidation occurs during discharge |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasNegativeElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5d299271_3f68_494f_ab96_3db9acdd3138
| elucidation | relates an electrochemical cell to the electrode at which oxidation occurs during discharge |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasPositiveElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8e9cf965_9f92_46e8_b678_b50410ce3616
| elucidation | relates an electrochemical cell to the electrode at which reduction occurs during discharge |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasPositiveElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8e9cf965_9f92_46e8_b678_b50410ce3616
| elucidation | relates an electrochemical cell to the electrode at which reduction occurs during discharge |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasReferenceElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5eb197ea_0c3e_4ea2_8392_81152ee91515
| elucidation | relates an electrochemical cell or measurement setup to the electrode that provides a stable reference potential |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasReferenceElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5eb197ea_0c3e_4ea2_8392_81152ee91515
| elucidation | relates an electrochemical cell or measurement setup to the electrode that provides a stable reference potential |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasSeparator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9317be62_e602_4343_a72d_02c87201b9f6
| elucidation | relates a system to a separator that physically divides electrodes while allowing ionic transport |
| subclassOf | owl.ObjectProperty, hasComponent |
hasSeparator#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_9317be62_e602_4343_a72d_02c87201b9f6
| elucidation | relates a system to a separator that physically divides electrodes while allowing ionic transport |
| subclassOf | owl.ObjectProperty, hasComponent |
hasSolute#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8784ef24_320b_4a3d_b200_654aec6c271c
| subclassOf | owl.ObjectProperty, hasComponent |
hasSolute#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8784ef24_320b_4a3d_b200_654aec6c271c
| subclassOf | owl.ObjectProperty, hasComponent |
hasSolvent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_808e94df_e002_4e64_a6db_182ed75078c6
| subclassOf | owl.ObjectProperty, hasComponent |
hasSolvent#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_808e94df_e002_4e64_a6db_182ed75078c6
| subclassOf | owl.ObjectProperty, hasComponent |
hasTerminationParameter#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e6a7d617_a581_4782_8374_37d3305e0258
| elucidation | a property that links a task to a parameter that determines the termination condition for that task |
| subclassOf | owl.ObjectProperty, hasInput |
hasTerminationParameter#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_e6a7d617_a581_4782_8374_37d3305e0258
| elucidation | a property that links a task to a parameter that determines the termination condition for that task |
| subclassOf | owl.ObjectProperty, hasInput |
hasTestEquipment#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_52702560_2034_4369_ab7f_28e8bb32680c
| elucidation | a property linking an electrochemical test to the equipment that is used to carry out the test. |
| subclassOf | owl.ObjectProperty, hasInput |
hasTestEquipment#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_52702560_2034_4369_ab7f_28e8bb32680c
| elucidation | a property linking an electrochemical test to the equipment that is used to carry out the test. |
| subclassOf | owl.ObjectProperty, hasInput |
hasTestObject#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5998f7c1_d8e3_4c55_8170_c358e1b0c93c
| elucidation | a property linking an electrochemical test to the object being tested |
| subclassOf | owl.ObjectProperty, hasInput |
hasTestObject#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_5998f7c1_d8e3_4c55_8170_c358e1b0c93c
| elucidation | a property linking an electrochemical test to the object being tested |
| subclassOf | owl.ObjectProperty, hasInput |
hasWorkingElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c76dbfeb_f3d9_44d4_9cbe_0946924f311f
| elucidation | relates an electrochemical cell or measurement setup to the electrode at which the primary reaction of interest occurs |
| subclassOf | owl.ObjectProperty, hasElectrode |
hasWorkingElectrode#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_c76dbfeb_f3d9_44d4_9cbe_0946924f311f
| elucidation | relates an electrochemical cell or measurement setup to the electrode at which the primary reaction of interest occurs |
| subclassOf | owl.ObjectProperty, hasElectrode |
includeDocs#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7104c722_d5a5_4bff_ba2f_37e229bb6cce
| subclassOf | owl.AnnotationProperty, rdf-schema.comment |
includeDocs#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_7104c722_d5a5_4bff_ba2f_37e229bb6cce
| subclassOf | owl.AnnotationProperty, rdf-schema.comment |
indigoLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8377c1f7_2b09_4632_9a4d_bb318438adbb
| elucidation | the label of a term used in resources from Indigo |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
indigoLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_8377c1f7_2b09_4632_9a4d_bb318438adbb
| elucidation | the label of a term used in resources from Indigo |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
maccorLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_097ea615_c209_4edd_a1a1_5eea71d98fe8
| elucidation | the label of a term used in resources from Maccor |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
maccorLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_097ea615_c209_4edd_a1a1_5eea71d98fe8
| elucidation | the label of a term used in resources from Maccor |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
newareLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_811aafc3_0c6d_470e_b67e_ff5c82992609
| elucidation | the label of a term used in resources from Neware |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
newareLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_811aafc3_0c6d_470e_b67e_ff5c82992609
| elucidation | the label of a term used in resources from Neware |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
novonixLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ea9f4dba_8ca2_419e_9cdf_ce54e9ebbd46
| elucidation | the label of a term used in resources from Novonix |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
novonixLabel#
IRI: https://w3id.org/emmo/domain/electrochemistry#electrochemistry_ea9f4dba_8ca2_419e_9cdf_ce54e9ebbd46
| elucidation | the label of a term used in resources from Novonix |
| subclassOf | owl.AnnotationProperty, core.hiddenLabel |
End of Document.
